Electrolyte capable of being used for lithium metal secondary battery and application of electrolyte
A secondary battery and electrolyte technology, applied in secondary batteries, secondary battery repair/maintenance, organic electrolytes, etc., can solve the problems of shortening battery life, easily generating dendrites, affecting battery coulomb efficiency, etc. The effect of deposition overpotential, simple preparation steps and excellent safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
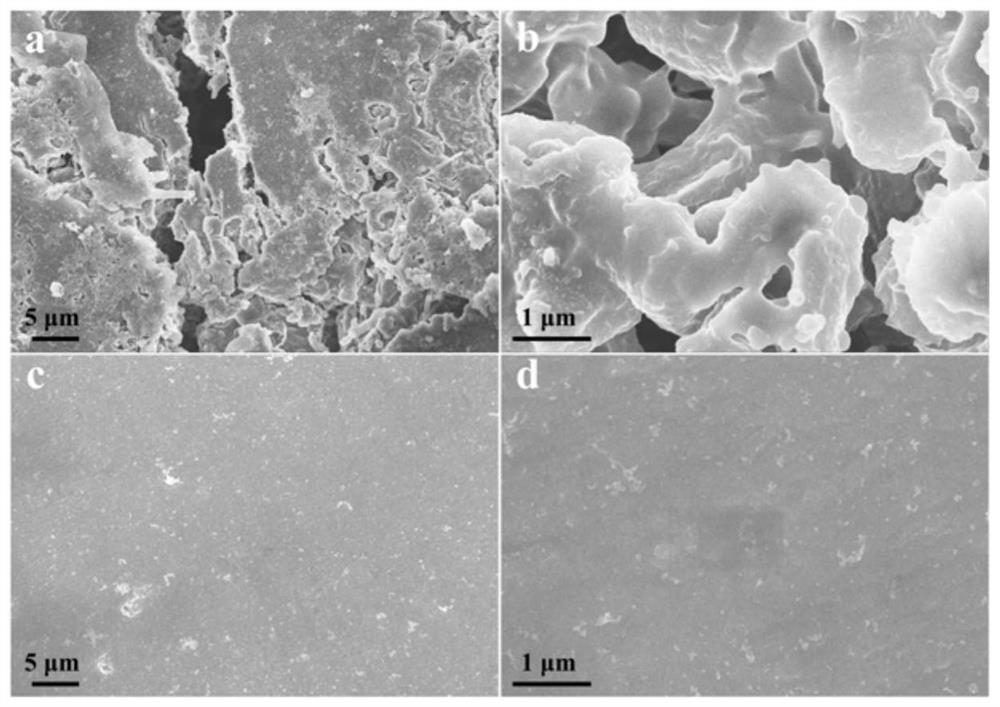
[0040] In a glove box filled with inert gas (argon), add phenylphosphonic acid to carbonate electrolyte (0.01MLiAsO 6dissolved in EC / DMC / FEC (volume ratio 1:1:1, total volume 100ml), made into an electrolyte solution with a mass fraction of phenylphosphonic acid of 0.1%, and stirred for 10 minutes to obtain the phenylphosphonic acid-containing Phosphonic acid electrolyte. The electrolyte prepared by this method is used to assemble Li||Li symmetrical battery, and the separator is PE. After the battery was cycled for 50 cycles, the surface of the electrode without phenylphosphonic acid in the electrolyte appeared obvious dendrite morphology ( figure 1 ), while the electrode surface with phenylphosphonic acid added to the electrolyte remained flat after 50 cycles. in figure 1 a and figure 1 b is the surface morphology of the electrode without adding phenylphosphonic acid, figure 1 c and figure 1 d is the surface morphology of the electrode added with phenylphosphonic acid. ...
Embodiment 2
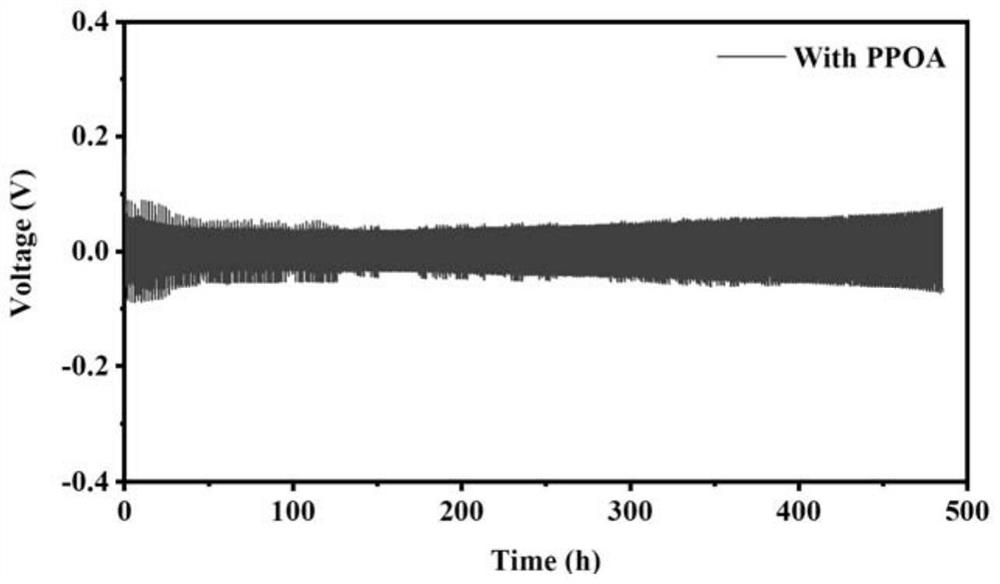
[0042] In a glove box filled with inert gas (helium), add phenylphosphonic acid to ester electrolyte (0.5M LiPF 6 dissolved in EC / DMC / FEC (volume ratio 1:1:1, total volume 100ml), made into an electrolyte solution with a mass fraction of phenylphosphonic acid of 0.5%, and stirred for 30 minutes to obtain the phenylphosphonic acid-containing Phosphonic acid electrolyte. The electrolyte solution prepared by this method is used to assemble Li||Li symmetrical battery, and the separator is PE / PP. figure 2 After assembling the battery for the above-mentioned electrolyte solution containing 0.5wt.% phenylphosphonic acid, at a current density of 0.5mA / cm 2 , the deposition capacity is 0.5mAh / cm 2 Under the same conditions, the voltage-time diagram of the battery is stable for 480 hours, the polarization voltage is lower than 60mV, and the charge-discharge curve is stable.
Embodiment 3
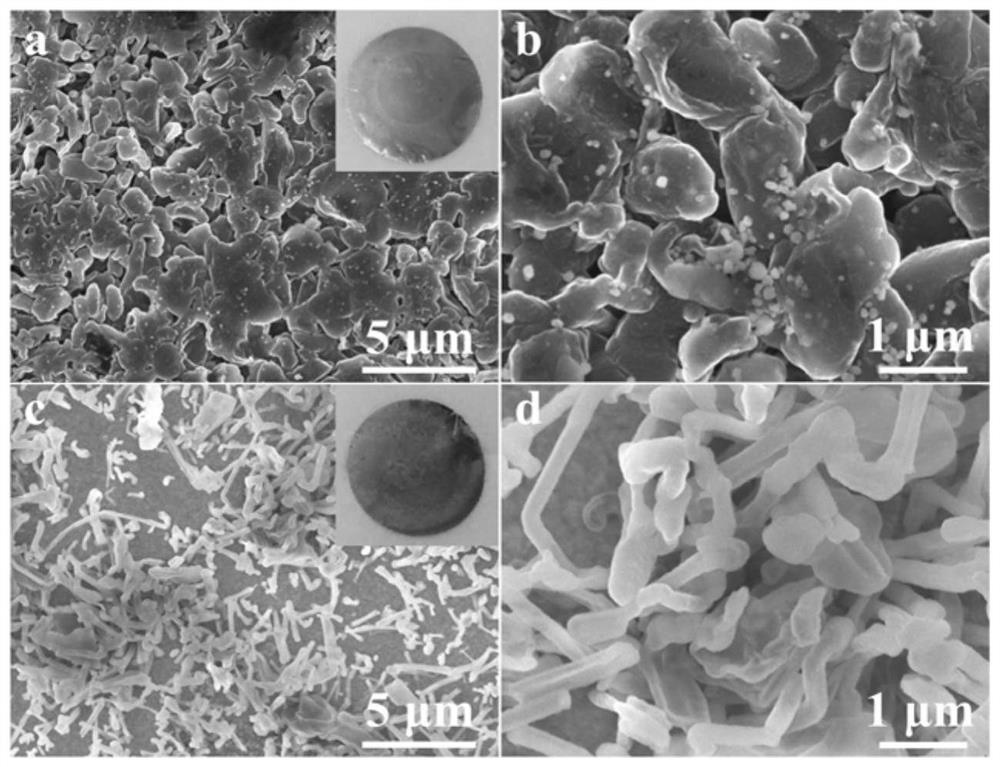
[0044] Under the protection of an inert gas (helium), add phenylphosphonic acid to the ester electrolyte (1M LiBF4 dissolved in EMC / DMC / DEC, volume ratio 1:1:1, total volume 100ml) to form phenyl The solution with a mass fraction of phosphonic acid of 1% was stirred for 1 h until it was completely dissolved, and the electrolyte solution containing phenylphosphonic acid described in the present invention was obtained. The electrolyte prepared by this method is used to assemble Li||Cu batteries, with PP film as the separator, and the battery operates at 1mA / cm 2 Under the current density of , after one hour of discharge, the lithium deposited on Cu foil as image 3 shown. The results of SEM showed that the deposition of lithium on the copper foil was very uniform after adding the phenylphosphonic acid additive, and the deposited lithium showed a spherical shape; the lithium deposition on the copper foil without the additive was very uneven and showed a rod shape.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com