Novel method for preparing 3-aminopyrazole-4-formamide hemi-sulfate
A technology of hemisulfate and aminopyrazole, applied in the field of pharmaceutical synthesis, can solve the problems of long reaction steps, compressed allopurinol profit space, high cost of malononitrile, etc., and achieves mild reaction conditions, simple preparation method and production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 12-cyano group-3-(dimethylamino)acrylamide
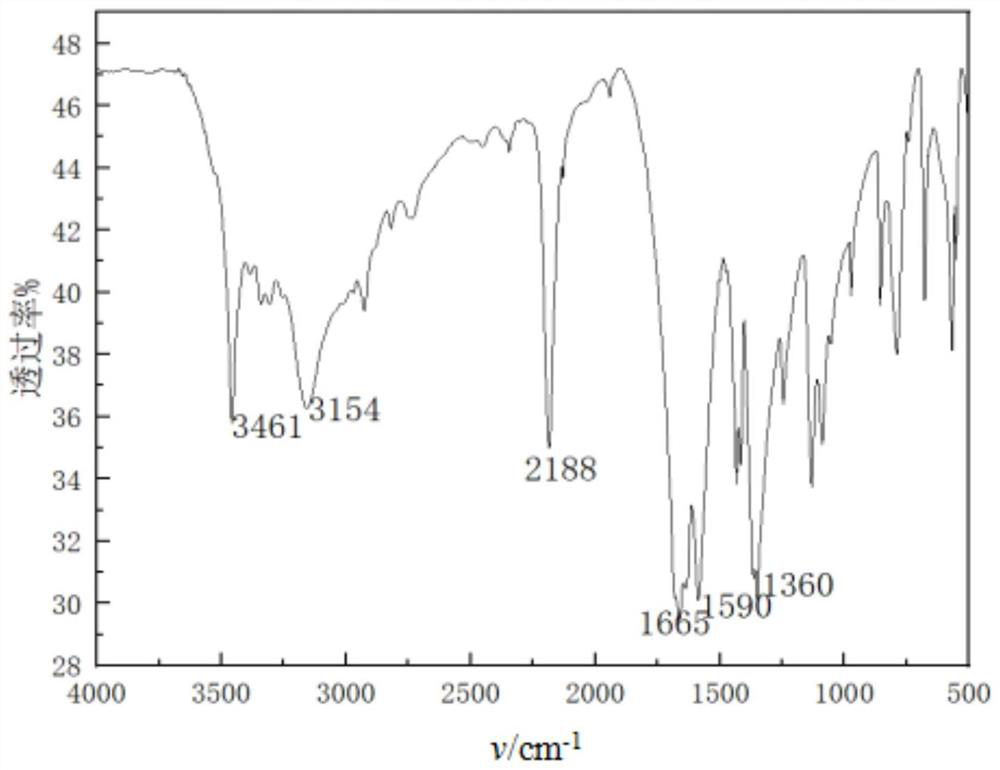
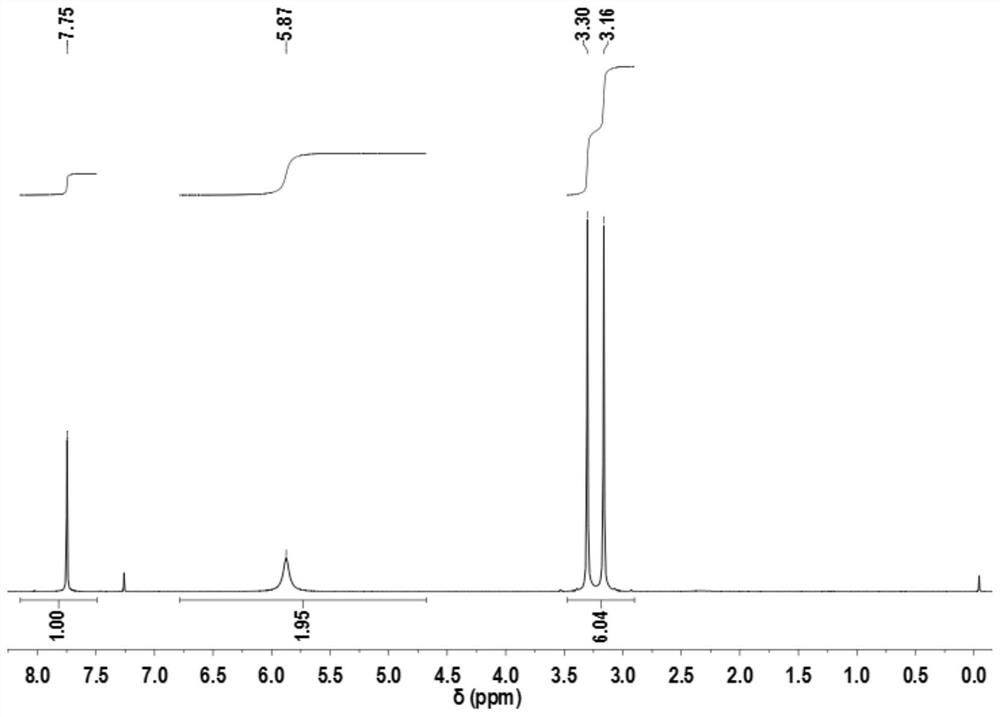
[0022] Dissolve 2.5g (0.03mol) of cyanoacetamide in 30mL (0.35mol) of 1,4-dioxane, then add 3.9g (0.033mol) of N,N-dimethylformamide dimethyl acetal, in Reaction at 50°C for 3h. After the reaction was complete as monitored by TLC, the 1,4-dioxane was evaporated under reduced pressure, and the obtained solid was washed with 5 mL (0.06 mol) of cold 1,4-dioxane, and dried to obtain 4.03 g of a white product, with a yield of 96%. . mp: 145-147°C. IR(KBr), ν / cm -1 : 3461, 3154 (-NH 2 ); 2188 (-CN); 1665 (C=O); 1559 (C=C). 1 HNMR (DMSO-d 6 ): 3.23(s,6H); 5.87(s,2H); 7.75(s,1H).
Embodiment 23
[0023] Synthesis of embodiment 23-aminopyrazole-4-carboxamide hemisulfate
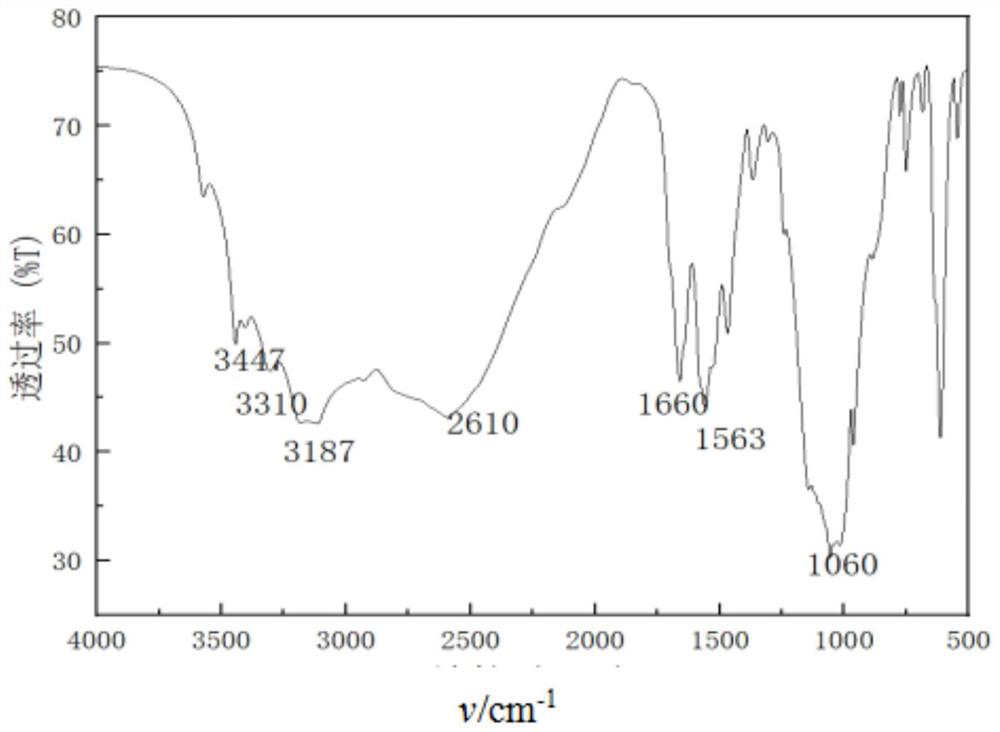
[0024] Add 40mL (0.77mol) of absolute ethanol and 2.0g (0.04mol) of hydrazine hydrate to 4.17g (0.03mol) of 2-cyano-3-(dimethylamino)acrylamide, and then reflux at 80°C for 5h. After TLC monitors that the reaction is complete, adjust the pH 1-2 of the solution with 50% sulfuric acid by mass percentage, continue to stir for 20 min, filter with suction, and wash the filter cake successively with 5 mL of water (0.28 mol) and 5 mL (0.07 mol) of acetone to obtain a white solid powder 3 -Aminopyrazole-4-carboxamide hemisulfate 3.26 g, yield 93%. mp: 224-226°C. IR(KBr), ν / cm -1 : 3447, 3310, 3187(N-H); 1660(C=O); 1563(C=C); 1 HNMR (DMSO-d 6 )δ8.05(s,4H,NH 2 ).
Embodiment 3
[0025] Example 3 One-step synthesis of 3-aminopyrazole-4-carboxamide hemisulfate
[0026] Dissolve 2.5g (0.03mol) of cyanoacetamide in 104mL (1.20mol) of 1,4-dioxane, then add 7.08g (0.06mol) of N,N-dimethylformamide dimethyl acetal, in Reaction at 40°C for 2h. After the completion of the reaction as monitored by TLC, the solvent was distilled off under reduced pressure. The obtained solid was washed with 7.6mL (0.09mol) of cold 1,4-dioxane, and then 52.5mL of absolute ethanol (0.9mol) and 4.5g (0.09mol) of hydrazine hydrate were added to the above reaction system, and refluxed at 70°C 5h. After TLC monitors that the reaction is complete, adjust the pH of the solution to 1-2 with 50% sulfuric acid by mass percent, continue to stir for 25 min, filter with suction, and wash the filter cake successively with 4.3 mL of water (0.24 mol) and 4.4 mL (0.06 mol) of acetone to obtain a white solid Powdered 3-aminopyrazole-4-carboxamide hemisulfate 4.68g, yield 88.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com