Double-label immunodetection method containing internal reference and application of double-label immunodetection method
A detection method and immunoassay technology, applied in the field of double-labeled immunoassay with internal reference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of the double-labeled kit of embodiment 1cTnI and the establishment of detection method
[0067] Taking the detection of troponin I (cTnI) as an example, the preparation method of the double-labeled kit for detecting cTnI is as follows:
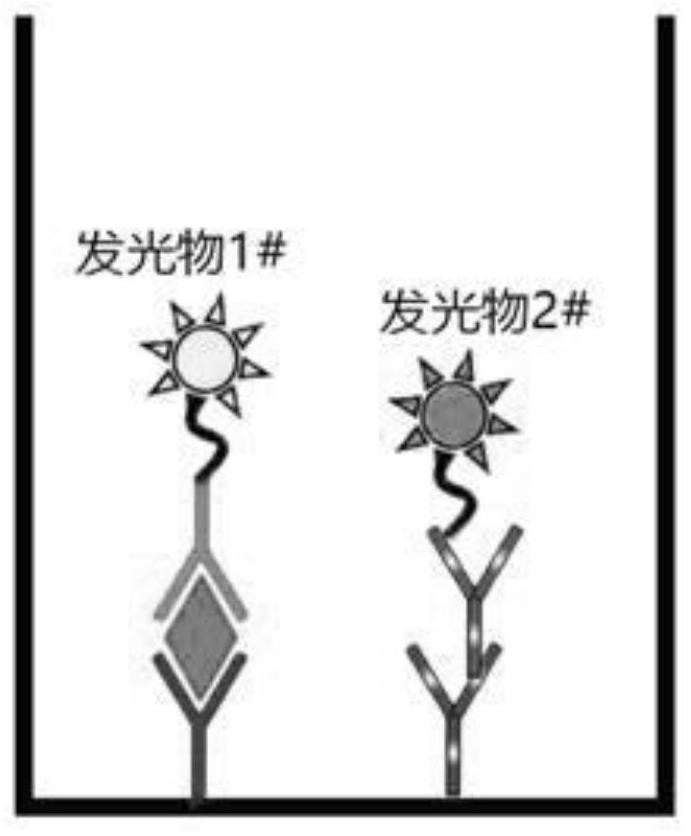
[0068] Step 1: Preparation of cTnI antibody and chicken IgY coated plates. Add 4 μg / mL cTnI antibody (Fepone Bio cTnI-MCAB-29, epitope 24-40aa) and 1 μg / mL chicken IgY (Biolab F050315) to the coating solution at the same time, mix for half an hour, take Add 100 μl to the coated plate and let it stand at 4°C for 22 hours. After washing the coated plate, add blocking solution, block at 37°C for 3 hours, and dry it for later use.
[0069] Step 2: Preparation of europium-labeled cTnI antibody. The β-diketone chelating agent europium and cTnI antibody (Fepeng biological cTnI-MCAB-22, epitope 86-90aa) were routinely labeled. After purification, the europium-labeled cTnI antibody was obtained with a concentration of 0.2 mg...
Embodiment 2
[0090] Example 2 Comparison of detection precision of cTnI double-labeled kit
[0091] Use the double-labeled kit for detecting cTnI of the present invention and the single-labeled detection kit for using self-made europium-labeled cTnI antibody (compared with the cTnI double-labeled kit of the present invention) to detect two high and low quality controls (self-made cTnI quality control) Products, the concentration is 2500pg / mL and 100pg / mL, antigen: sea peptide biological recombinant human cardiac troponin I8RTI7; quality control dilution: 100mM Tris-HCl, 5% BSA, 0.1% P300 preservative, PH7.8 ), wherein the double-labeled kit adopts the detection method described in Example 1, and the single-labeled detection kit is operated according to the detection method of the conventional single-labeled kit, and the detection is repeated 10 times, and the precision is calculated, and the results are as follows:
[0092] table 2-1
[0093]
[0094] Table 2-2
[0095]
[0096] Re...
Embodiment 3
[0098] Embodiment 3 internal reference marker concentration selection
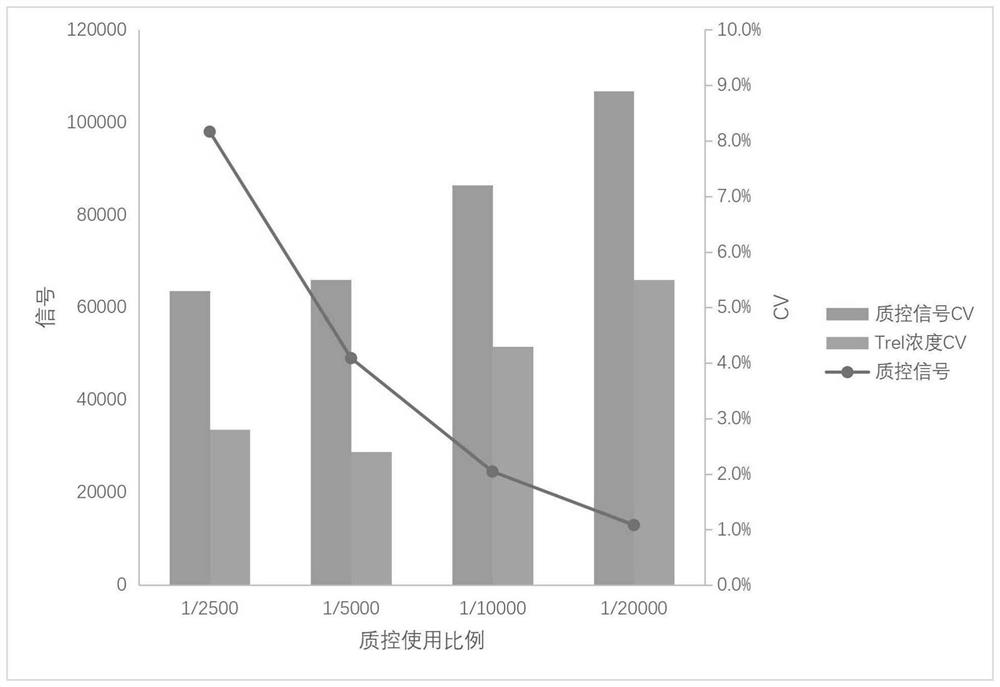
[0099] 1) Selection of quality control concentration: Use ratios of samarium-labeled goat anti-chicken IgY (0.2mg / mL) to 1:2500, 1:5000, 1:10000, 1:20000 respectively with europium-labeled cTnI antibody (0.2mg / mL)1 : 1000 At the same time, add labeling buffer and mix well, as cTnI double labeling (reagent).
[0100] 2) Detection method:
[0101] Using these four double-labeled reagents, refer to the preparation method and detection method of the double-labeled kit described in Example 1, and simultaneously detect the serum samples from the same source.
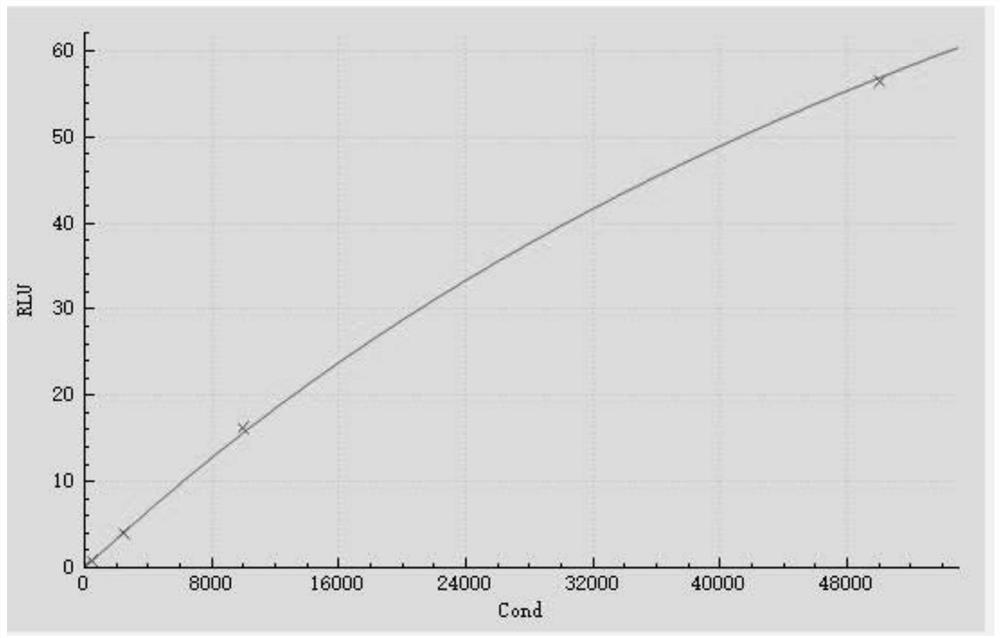
[0102] Eu will be detected separately 3+ Signal and Sm 3+ The signal is calculated as the relative signal value of the serum sample by the formula: (T-B1) / (C-B2). Substitute the relative signal value into the fitting curve, and inversely calculate the cTnI concentration value of the sample.
[0103] 3) Test results
[0104] Using the above 4 groups o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com