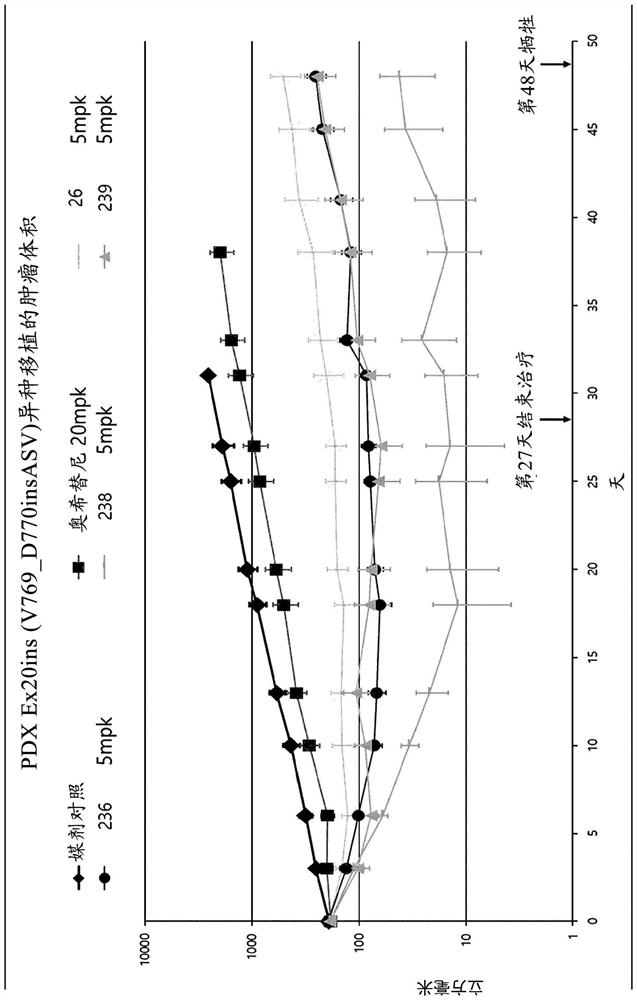
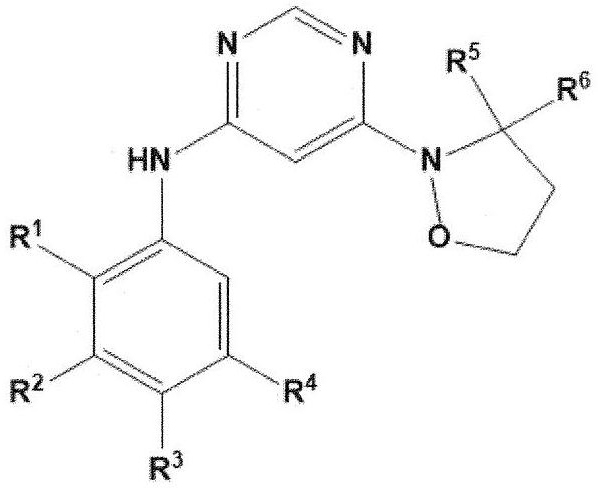
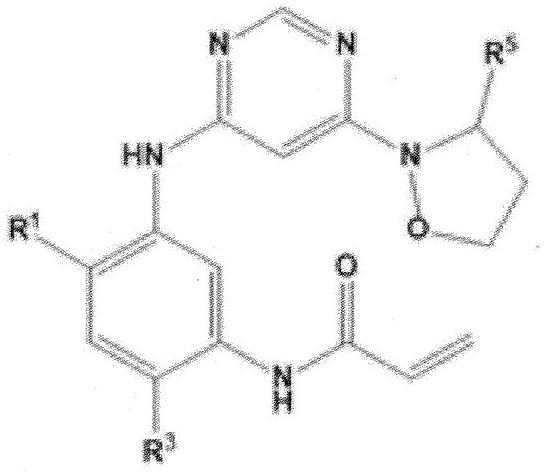
Heteroaryl derivative, method for producing same, and pharmaceutical composition comprising same as effective component
A technology of heteroaryl and compound, applied in the field of heteroaryl derivatives, can solve the problems such as the inability of therapeutic agents to exhibit efficacy and the inability of EGFR inhibitors to exert efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0207] Preparation of (S)-3-phenylisoxazolidine
[0208]
[0209] Procedure 1: Preparation of tert-butyl (R)-(3-hydroxy-3-phenylpropoxy)carbamate
[0210] Tert-butyl hydroxycarbamate (7.8 g, 58.6 mmol) was dissolved in dimethylformamide (140 ml), then sodium hydride (2.58 g, 64.5 mmol) was added thereto at 0° C., and the reaction was carried out for 30 minutes . Then, (R)-3-chloro-1-phenylpropan-1-ol (5 g, 29.3 mmol) dissolved in dimethylformamide (10 ml) was slowly added dropwise at 0 °C for 10 minutes, followed by Stir at room temperature for 72 hours. Aqueous ammonium chloride solution was added to the reaction mixture to terminate the reaction, followed by extraction with ethyl acetate and brine, whereby the organic layers were combined. The organic layer was dried over sodium sulfate, concentrated under reduced pressure, and purified by medium-pressure liquid chromatography (ethyl acetate / n-hexane), thereby obtaining the target compound (R)-(3-hydroxy-3-phenylpropa...
preparation example 2
[0218] Preparation of (R)-3-phenylisoxazolidine
[0219]
[0220] Preparation Example 2 was carried out in the same manner as Preparation Example 1, and the compounds of Examples shown in Table 1 below were used for synthesis.
[0221] MS (m / z): 150.08[M+1]+, UPLC retention time (min): 0.72
preparation example 3
[0222] Preparation of (R)-3-(3-fluorophenyl)isoxazolidine
[0223]
[0224] Procedure 1: Preparation of 3-fluoro-N-methoxy-N-methylbenzamide
[0225] 3-Fluorobenzoic acid (90 g, 642.35 mmol, 1 eq) was dissolved in pyridine (150 mL), and N-methoxymethylamine (75.19 g, 770.81 mmol, 1.2 eq, HCl) was added thereto. Then, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI; 147.77 g, 770.81 mmol, 1.2 eq) was added at 15°C. The reaction mixture was stirred at 50 °C for 30 minutes. As a result of TLC analysis (PE:EA=3:1 ), all starting material disappeared and new low polar spots were detected. The pyridine solvent was removed by concentration under reduced pressure, and the organic layer was extracted with dichloromethane (DCM; 500 mL), hydrochloric acid (500 mL, 2N), and brine (200 mL). The organic layer was dried over sodium sulfate and then concentrated under reduced pressure to obtain the target compound 3-fluoro-N-methoxy-N-methylbenzamide (110 g, 600.50 mmol, yield 93.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com