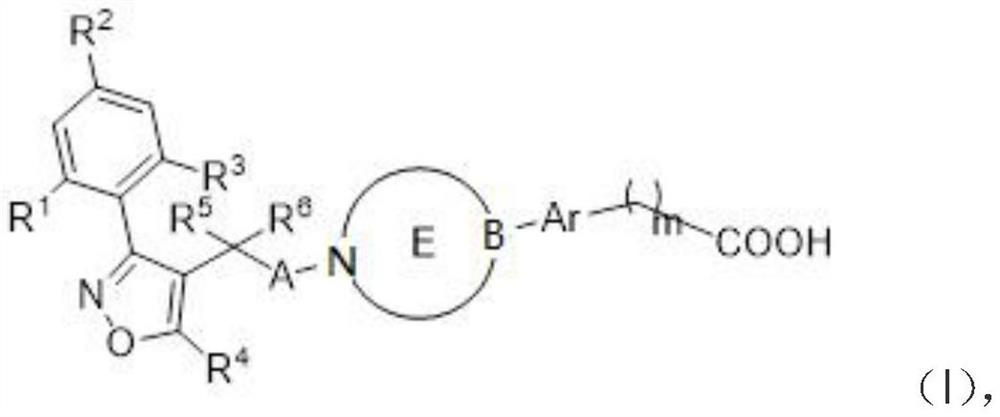
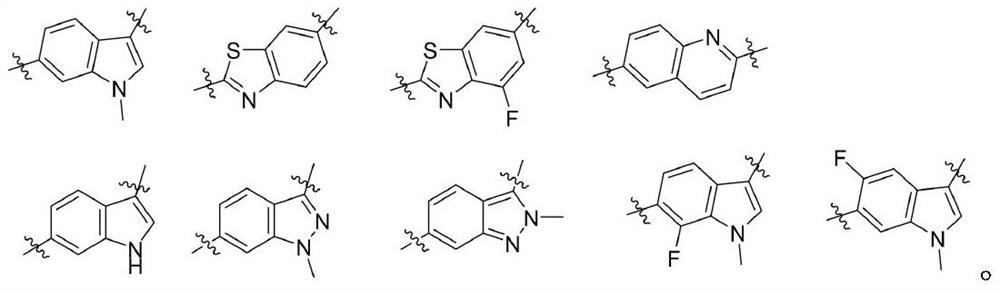
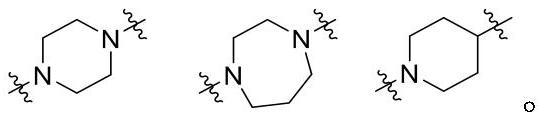
Compounds for modulating FXR
A technology of compounds, compositions, applied in the fields of medicinal chemistry, pharmacology and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0259] 6-(4-(2-(5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)ethyl)piperazin-1-yl)-1-methyl -1H-indole-3-carboxylic acid (I1)
[0260]
[0261]Step 1: Synthesis 4:
[0262]
[0263] Add K to a solution of compound 3 (31 g, 122.0 mmol) in acetonitrile (ACN, 300 mL) at room temperature 2 CO 3 (33.7 g, 244.0 mmol), followed by the addition of iodomethane (MeI) (43.3 g, 305.0 mmol). The reaction mixture was stirred overnight then concentrated. The residue was diluted with water, extracted with ethyl acrylate (EA), and the organic layers were combined, washed with brine, dried, concentrated and purified by silica gel column (heptane / EA=5:1) to give the desired product, compound 4 (12.1 g, white solid, 15% overall yield over three steps). LCMS: (ESI-MS): [M+H] + =268.0,270.0; 1 H NMR (400MHz, DMSO) δppm: 8.15(s,1H), 7.92(d, J=8.8Hz,1H), 8.84(s,1H), 7.38(s,1H), 3.85(s,3H), 3.80 (s,3H).
[0264] Step 2: Synthesis 5:
[0265]
[0266] Under nitrogen, compound 4 ...
Embodiment 2
[0298] 6-(4-(2-(5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazole-4-acetyl)ethyl)piperazin-1-yl)-1-methanol Amyl-1H-indole-3-carboxylic acid (I2)
[0299]
[0300] Step 1: Synthesis of 16:
[0301]
[0302] To a solution of compound 11 (460 mg, 1.62 mmol) in DCM (10 mL) was added triphenylphosphine (PPh3, 637 mg, 2.43 mmol). Then, CBr was added dropwise to the mixture 4 (805mg, 2.43mmol), and stirred at room temperature for 1 hour. After the reaction was completed, the reaction solution was concentrated and purified by silica gel column (heptane / EA=10:1) to obtain the desired product, compound 16 (360 mg, white solid, yield 64%). LCMS: (ESI-MS): [M+H]+=347.9; 1H NMR (300MHz, CDCl 3 ) δppm: 7.47-7.37 (m, 3H), 4.24 (s, 2H), 2.17-2.12 (m, 1H), 1.32-1.29 (m, 2H), 1.24-1.20 (m, 2H).
[0303] Step 2: Synthesis of 17:
[0304]
[0305] To a solution of compound 16 (320 mg, 0.92 mmol) in THF (5 mL) was added TBAF 1M / THF (1.84 mL, 1.84 mmol). Trimethylcyanosilane (TMSCN, 18...
Embodiment 3
[0316] 6-(4-(2-(3-(2,6-dichlorophenyl)-5-isopropylisoxazol-4-yl)ethyl)piperazin-1-yl)-1-methyl -1H-indole-3-carboxylic acid (I3)
[0317]
[0318] Step 1: Synthesis of 22
[0319]
[0320] According to the synthetic steps of 10, compound 21 was obtained. 1 H NMR (400MHz, DMSO-d6) δppm: 7.73-7.58 (m, 3H), 3.93-3.81 (m, 1H), 3.69 (s, 3H), 1.43 (d, J=6.8Hz, 6H).
[0321] Step 2: Synthesis 22:
[0322]
[0323] To a solution of compound 21 (6.0 g, 22 mmol) in THF (40 mL) was added lithium aluminum hydride (LAH) (88 mL, 88 mmol, 1 M in THF) at 0 °C. The reaction was stirred at room temperature for 2 hours, then 100 mL of 1N aqueous NaOH was added. The formed precipitate was filtered through celite and all solvent was removed in vacuo. The residue was purified by flash chromatography (PE:EA=1:2) to give product 22 (2.11 g white solid, yield: 33.5%). 1HNMR (400MHz, DMSO-d6): δppm: 7.71-7.51(m, 3H), 4.96-4.91(m, 1H), 4.22(d, J=4.8Hz, 2H), 3.41-3.35(m, 1H), 1.31 (d, J=6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com