Pseudo-ginseng quality detection method based on spectrum-activity relationship
A quality detection method and detection method technology, which are applied in the field of pharmaceutical inventions, can solve the problems of misjudgment of characteristic peaks, ratio of characteristic peaks of unconfirmed characteristic peaks relative to retention time, difficulty in determining the qualified range of medicinal materials, etc. The effect of accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
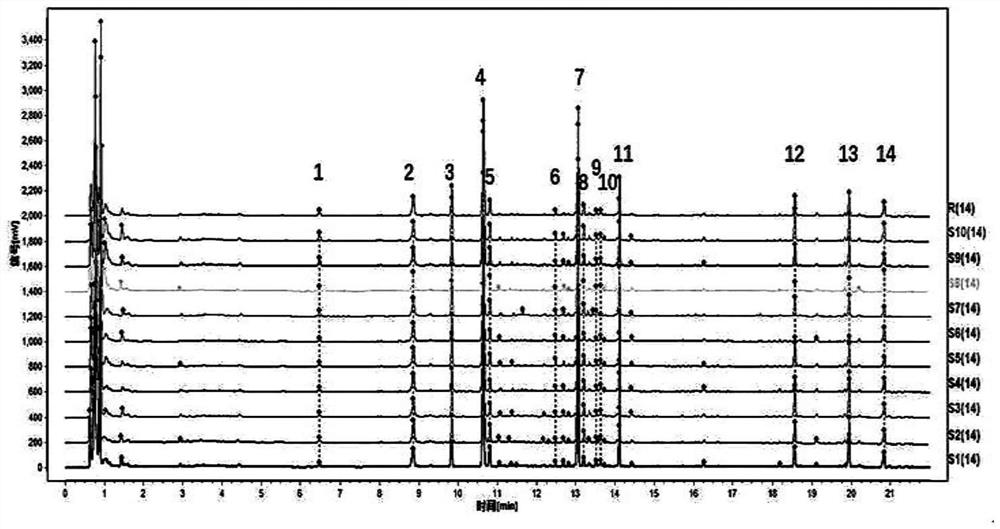
[0050] Fingerprint
[0051] Preparation of the mixed reference substance solution: Accurately weigh 10 mg each of the reference substances notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside RH1 and ginsenoside Rg2, respectively, add methanol solution to constant volume In a 10mL volumetric flask, the standard solution is obtained;
[0052] Preparation of the test solution: Accurately weigh 15.0 g of Panax notoginseng powder, add 7.7 times the amount of 66% ethanol to reflux for extraction for 1.5 hours, extract twice, and combine the filtrates; dilute the filtrate to a 200mL volumetric flask, and centrifuge at 13000r / min for 10min , through a 0.22μm microporous membrane to obtain the test solution.
[0053] Chromatographic conditions and system adaptability test: Chromatographic column: ACQUITY UPLC BEH Shield C18, chromatographic column specification: 2.1×100mm, 1.7μm; mobile phase: 0.1% phosphoric acid water as mobile phase A, ...
Embodiment 2
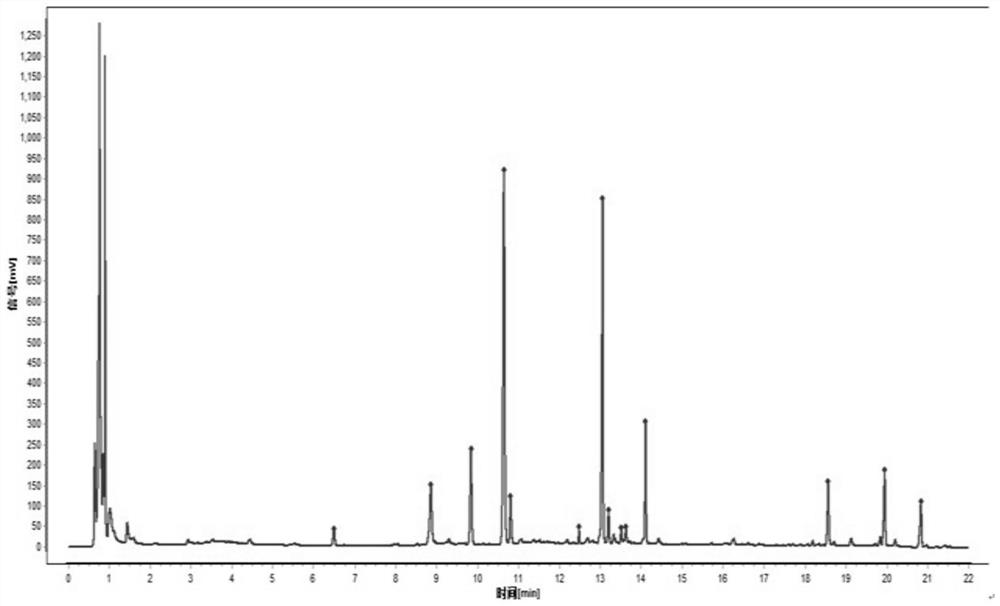
[0083] Fingerprint
[0084] Preparation of the mixed reference substance solution: Accurately weigh 10 mg each of the reference substances notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside RH1 and ginsenoside Rg2, respectively, add methanol solution to constant volume In a 10mL volumetric flask, the standard solution is obtained;
[0085] Preparation of the test solution: Accurately weigh 15.0 g of Panax notoginseng powder, add 7 times the amount of 60% ethanol to reflux for extraction for 1 hour, extract twice, and combine the filtrates; dilute the filtrate to a 200mL volumetric flask, and centrifuge at 13000r / min for 5min , through a 0.22 μm microporous membrane to obtain the test solution;
[0086] Chromatographic conditions and system adaptability test: Chromatographic column: ACQUITY UPLC BEH Shield C18, chromatographic column specification: 2.1×100mm, 1.7μm; mobile phase: 0.1% phosphoric acid water as mobile phase A, mobil...
Embodiment 3
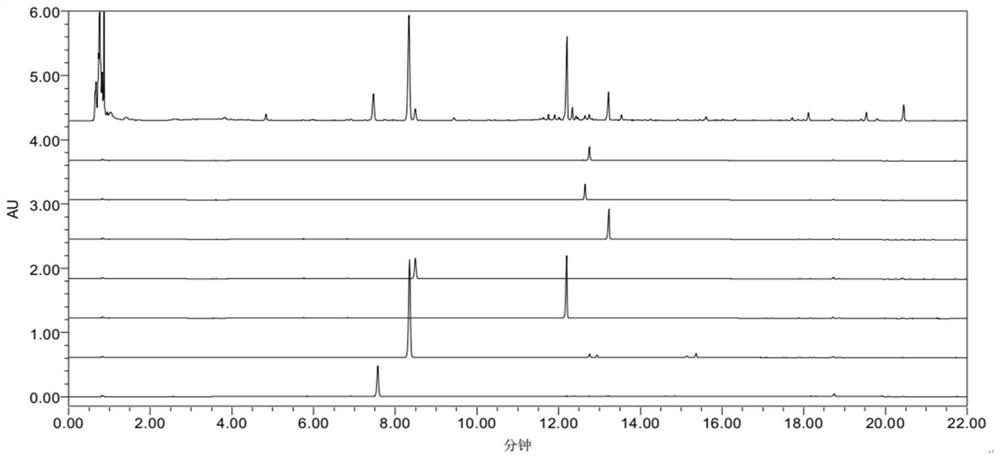
[0090] Fingerprint
[0091] Preparation of the mixed reference substance solution: Accurately weigh 10 mg each of the reference substances notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Re, ginsenoside Rd, ginsenoside RH1 and ginsenoside Rg2, respectively, add methanol solution to constant volume In a 10mL volumetric flask, the standard solution is obtained;
[0092] Preparation of the test solution: Accurately weigh 15.0 g of Panax notoginseng powder, add 8 times the amount of 70% ethanol to reflux for extraction for 2 hours, extract twice, and combine the filtrates; dilute the filtrate to a 200mL volumetric flask, and centrifuge at 13000r / min for 15min , through a 0.22 μm microporous membrane to obtain the test solution;
[0093] Chromatographic conditions and system adaptability test: Chromatographic column: ACQUITY UPLC BEH Shield C18, chromatographic column specification: 2.1×100mm, 1.7μm; mobile phase: 0.1% phosphoric acid water as mobile phase A, mob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com