Preparation method of p-nitrophenylacetone
A technology of nitropropiophenone and p-nitrophenylacetic acid, which is applied in the field of preparation of p-nitropropiophenone, can solve problems affecting product quality, difficult to remove by-product impurities, cumbersome purchase and storage procedures, etc., and achieve industrial production , the effect of good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
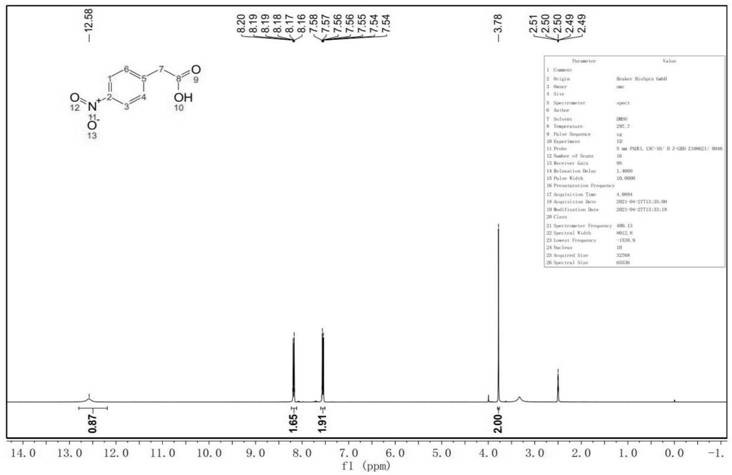
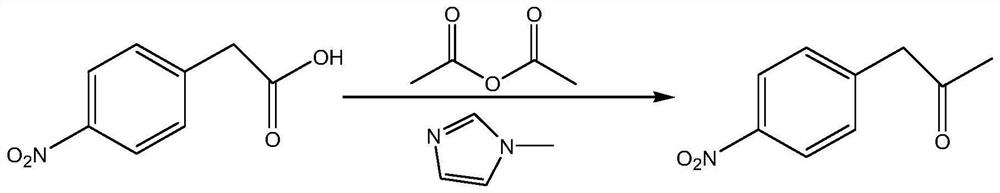
[0033] A kind of preparation method of p-nitropropiophenone, its synthetic route comprises:
[0034]
[0035] Adopt cheap and easy-to-get phenylacetonitrile as starting raw material, obtain p-nitropropiophenone through nitration, hydrolysis, acid chloride, condensation, deacidification, comprising the following steps:
[0036] S1. Nitration: Under the conditions of participation of nitrating agent and dehydrating agent, phenylacetonitrile is nitrated at 15-20°C. The nitrating agent is nitric acid with a concentration of 60-68%, and the catalyst is concentrated sulfuric acid. Phenylacetonitrile, nitrating agent And the molar ratio of the catalyst is 1:1.1-1.2:3.0-5.0;
[0037] S2. Hydrolysis: Complete the hydrolysis process at 100-105°C, precipitate and wash the solid, and obtain p-nitrophenylacetic acid crystals after recrystallization. The solvent used in the recrystallization process includes ethanol and deionized water. The amount of solvent used For 2 times the amount ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com