Liraglutide analogue and preparation method thereof
A technology of liraglutide and liraglutidyl, applied in the field of pharmaceutical peptides, can solve problems such as long action time, and achieve the effects of lowering blood sugar, reducing inflammation, and improving glucose metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
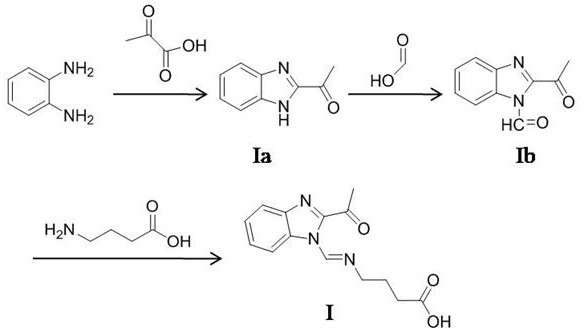
[0072] The synthetic method of formula I compound, the route of its synthetic method sees figure 1 ,include,
[0073] Step I-a, add 0.1 mol of o-phenylenediamine and 0.12 mol of pyruvic acid into 80 mL of 5 M HCl solution, stir and reflux at 85°C for 3.5 h, cool to room temperature after the reaction, and then use 3.2 M NaOH solution to adjust the pH to 9.5, suction filtration, collect solid, wash 3 times with distilled water, dry, obtain formula Ia compound, productive rate is 74.6%, the molecular formula of Ia compound is C 9 h 8 N 2 O, 1 HNMR (DMSO-d 6 , 300 MHz) δ: 12.18 (s, 1H, NH), 7.25-7.61(m, 4H, Ar-H), 2.85(m, 3H, CH 3 );
[0074] Step I-b, add 0.1 mol of the compound of formula Ia and 0.12 mol of formic acid into 100 mL of acetonitrile, stir and reflux at 85°C for 4 h, and distill under reduced pressure to obtain the compound of formula Ib with a yield of 71.5%. The molecular formula of compound Ib is C 10 h 8 N 2 o 2 , 1 HNMR (DMSO-d 6 , 300 MHz) δ: 9.55...
Embodiment 2
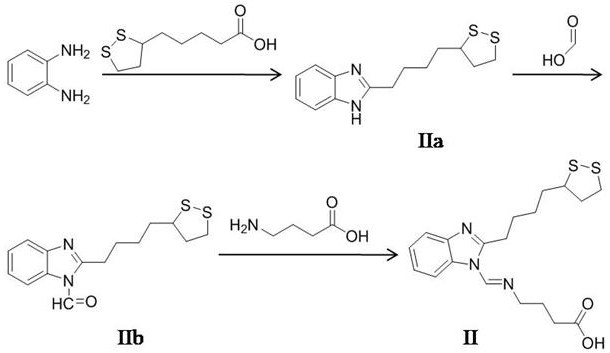
[0078] The synthetic method of formula II compound, the route of its synthetic method sees figure 2 ,include
[0079] Step II-a, add 0.1 mol o-phenylenediamine and 0.12 mol lipoic acid into 80 mL 5 M HCl solution, stir and reflux at 85°C for 3.5 h, cool to room temperature after the reaction, and then adjust with 3.2 M NaOH solution pH to 9.5, suction filtration, collect solid, wash 3 times with distilled water, dry, obtain formula IIa compound, productive rate is 72.8%, the molecular formula of IIa compound is C 14 h 18 N 2 S 2 , 1 HNMR (DMSO-d 6 , 300 MHz) δ: 12.36 (s, 1H, NH), 7.29-7.64(m, 4H, Ar-H), 2.97(t, 2H, CH 2 ), 2.52-2.65(t, 3H, S-CH, S-CH 2 ), 1.79-1.94(t, 2H, CH 2 ),1.25-1.68(t, 6H, CH 2 );
[0080] Step II-b, add 0.1 mol of the compound of formula IIa and 0.12 mol of formic acid into 100 mL of acetonitrile, stir and reflux at 85°C for 4 h, and distill under reduced pressure to obtain the compound of formula IIb with a yield of 71.2%, the molecular form...
Embodiment 3
[0084] A preparation method of liraglutide analogue-I, comprising,
[0085] Add 1 mmol liraglutide, 2.4 mmol formula I compound, 1.3 mmol EDCI and 0.25 mmol DMAP into 220 mL dichloromethane, react at room temperature, TLG traces the reaction process, after the reaction, the solvent is distilled off under reduced pressure, and then Slowly pour into 100 mL of 1.5 M HCl solution, extract 3 times with 50 mL of dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, and distill off the solvent under reduced pressure to obtain liraglutide analogue-I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com