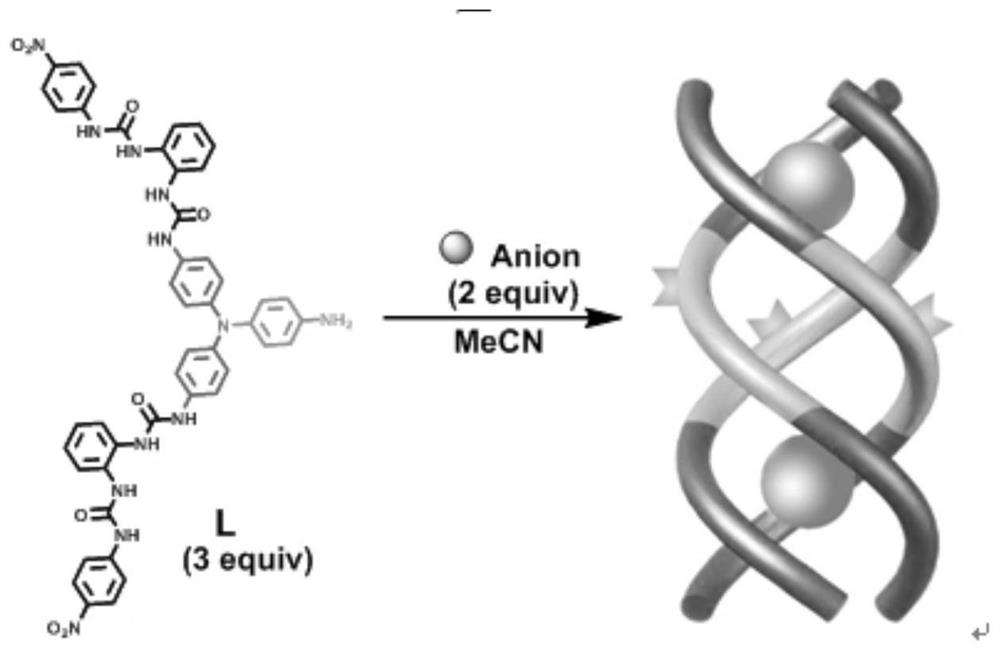
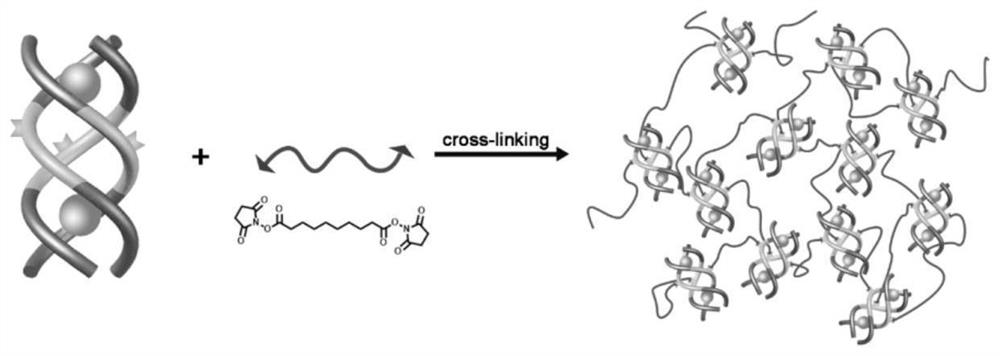
Anion coordination self-assembly supramolecular gel synthesis method and application
A supramolecular gel, self-assembly technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of large external influence, large radius, low charge density, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
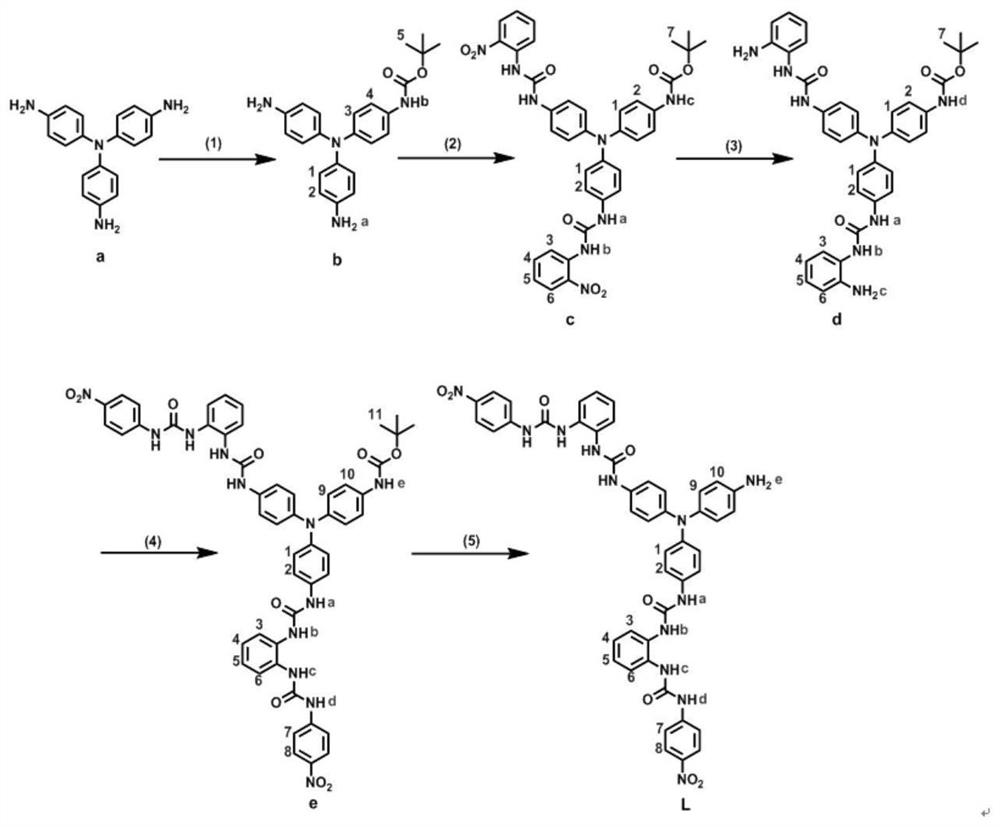
[0040] Synthesis and characterization of compound b:
[0041] Will (Boc) 2O (0.75g, 3.4mmol) tetrahydrofuran solution was slowly added dropwise to compound a (1g, 3.4mmol) in tetrahydrofuran solution (20mL), and reacted overnight in ice bath. After the reaction, the product was separated by silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 1:1), and the product was collected and rotary evaporated to obtain product b as a light pink solid with a yield of 50%. 1 H NMR (400MHz, DMSO-d 6 ,ppm): δ8.99(s,1H,Hb),7.14(d,J=8Hz,2H,H4),6.69(d,J=8Hz,4H,H1),6.58(d,J=8Hz,2H ,H3),6.48(d,J=8Hz,4H,H2),4.87(s,4H,Ha),1.44(s,9H,H5). 13 C NMR (100MHz, DMSO-d 6 , ppm): δ153.0, 144.7, 137.0, 131.2, 126.1, 119.5, 119.1, 114.8, 78.5, 28.2. ESI-MS m / z, found 390.2010, calcd for C 23 h 26 N 4 o 2 [M+H] + 391.2129.
Embodiment 2
[0043] Synthesis and characterization of compound c:
[0044] Compound b (0.5g, 1.28mmol) and o-nitrophenylisocyanate (0.46g, 2.81mmol) were dissolved in 5mL of tetrahydrofuran and added to a 50mL three-necked flask, heated and stirred at 50°C, the solution gradually turned dark red orange, Three hours later, the plate detection reaction was completed. The reaction liquid was rotary evaporated to about 2 mL, and diethyl ether was added to precipitate an orange-red solid compound c, which was filtered and dried with a yield of 98%. 1 H NMR (400MHz, DMSO-d 6 ,ppm): δ9.77(s,2H,Hb),9.57(s,2H,Ha),9.26(s,1H,Hc),8.31(d,J=8Hz,2H,H3),8.08(d, J=8Hz, 2H, H6), 7.69(t, J=8Hz, 2H, H4), 7.40(d, J=8Hz, 6H, H2), 7.19(t, J=8Hz, 2H, H5), 6.93( d,J=8Hz,6H,H1),1.47(s,9H,H7). 13 C NMR (100MHz, DMSO-d 6 , ppm): δ152.8, 151.8, 142.6, 141.9, 137.4, 135.1, 135.0, 134.6, 133.8, 125.4, 124.2, 123.5, 122.4, 122.0, 120.0, 119.6, 78.8, 28.1. ESI-MS m / z.22 957 calcd for C 37 h 34 N 8 o 8 [M+H] + 7...
Embodiment 3
[0046] Synthesis and characterization of compound d:
[0047] Add 3mL of hydrazine hydrate dropwise into 20mL of ethanol suspension containing compound c (0.7g, 1mmol) and Pd / C (70mg), react at 50°C for 5min, and wait for the orange-red particles in the solution to disappear, spot plate detection The reaction is over. The Pd / C was filtered out with diatomaceous earth, and the obtained filtrate was subjected to rotary evaporation, leaving a small amount of mother liquor, adding water to precipitate a white solid d, and the yield was 92%. 1 H NMR (400MHz, DMSO-d 6 ,ppm): δ9.22(s,H,Hd),8.65(s,2H,Hb),7.66(s,2H,Ha),7.32(d,J=8Hz,8H,H2 / H3),6.89( d,J=8Hz,6H,H1),6.83(t,J=8Hz,2H,H5),6.74(d,J=8Hz,2H,H6),6.26(t,J=8Hz,2H,H4), 4.75(s,4H,Hc),1.46(s,9H,H7). 13 C NMR (100MHz, DMSO-d 6 , ppm): δ153.2, 152.9, 142.3, 141.8, 140.8, 134.9, 134.2, 124.9, 124.3, 123.8, 123.6, 123.5, 119.4, 116.9, 115.9, 78.8, 28.2. ESI-MS m / z, found 697.28 for 02, C 37 h 38 N 8 o 4 [M+H] + 697.2648.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com