Synthesis method and intermediate of brexpiprazole
A technology of epipiprazole and a synthesis method, applied in the field of pharmaceutical raw material drug synthesis, can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of 7-(4-chlorobutoxy)-3,4-dihydro-2(1H)-quinolinone
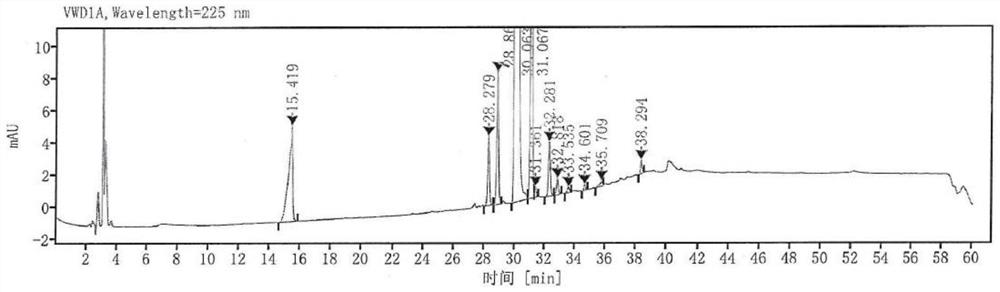
[0037] 7-hydroxy-3,4-dihydro-2(1H)-quinolinone (200g, 1.23mol), 1-bromo-4-chlorobutane (631g, 3.68mol) and potassium carbonate (254g, 1.84mol ) into a 5L three-neck flask in DMF (800mL) for mixing and stirring, then stirred at 25°C for 30h, and the suspension gradually became thicker and thicker. Purified water (1600mL) was slowly poured into the above-mentioned 5L three-neck flask with stirring, a large amount of solids were precipitated, and the stirring was continued for 30min, followed by suction filtration, and the solids were washed with purified water. Transfer the solid obtained above to a 1L three-necked flask, add methyl tert-butyl ether (400 mL) to beat and stir for 1 h, then filter with suction, and dry the obtained solid under vacuum at 50°C to obtain an off-white solid (258 g), with a yield of about 83.6% . HPLC detection (see figure 1 ) with a purity of about 96.14% (the content...
Embodiment 2
[0040] Embodiment 2: Synthesis of 7-(4-chlorobutoxy)-quinolin-2-one
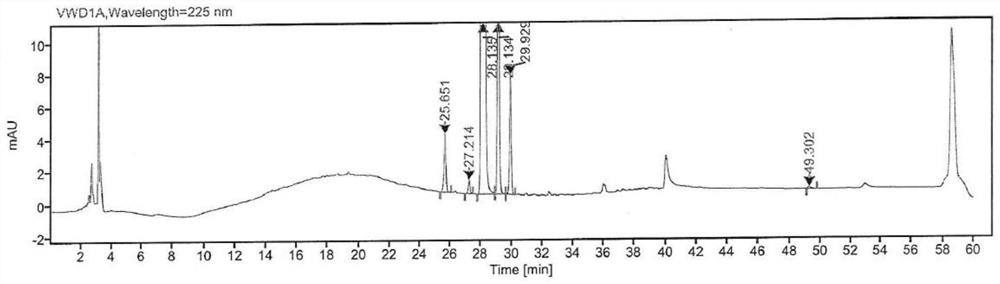
[0041] Put 7-(4-chlorobutoxy)-3,4-dihydro-2(1H)-quinolinone (150g, 0.59mol), THF (750mL) into a 1L three-necked flask, stir at room temperature and then slowly batch DDQ (161 g, 0.71 mol) was added and stirring was continued for 18 h. The above reaction solution was slowly poured into water (750 mL) for quenching, then aqueous sodium bicarbonate solution (1.57 mol / L, 750 mL) was slowly added under stirring, and stirred for 1 h. After suction filtration, the obtained solid was washed with water and dried under vacuum at 50° C. to obtain a light yellow solid (122 g) with a yield of 82.1%. HPLC detection (see figure 2 ) with a purity of about 97.43% (wherein the 7-(4-bromobutoxy)-quinolinone content is 1.67%).
[0042] Table 2: Extraction of the HPLC test result data of the intermediate of this embodiment
[0043]
Embodiment 3
[0044] Example 3: Synthesis of 1-N-tert-butoxyformyl-7-(4-chlorobutoxy)-quinolin-2-one
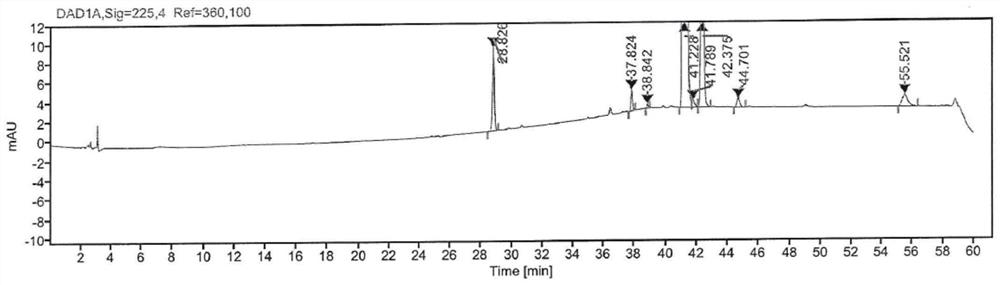
[0045] Mix 7-(4-chlorobutoxy)-quinolin-2-one (40.2g, 160mmol) and DMAP (1.9g, 16mmol) in dichloromethane (200mL), add dicarbonate dicarbonate dropwise under stirring at room temperature Tert-butyl ester (70.0g, 320mmol), dichloromethane (100mL) solution, followed by reaction at 35°C for 1h. The above dichloromethane reaction solution was washed with water (100ml×3), and then dried over anhydrous sodium sulfate. After filtration, dichloromethane was distilled off under reduced pressure at 35°C, and the resulting solid was stirred with methyl tert-butyl ether (400 mL) for 1 h. Filtration and washing of the solid with methyl tert-butyl ether and drying in vacuo afforded a white solid (44.9 g) in about 79.8% yield. HPLC detection (see image 3 ) with a purity of about 97.88% (the content of 1-N-tert-butoxyformyl-7-(4-bromobutoxy)-quinolin-2-one is 1.55%).
[0046] Table 3: Extraction of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com