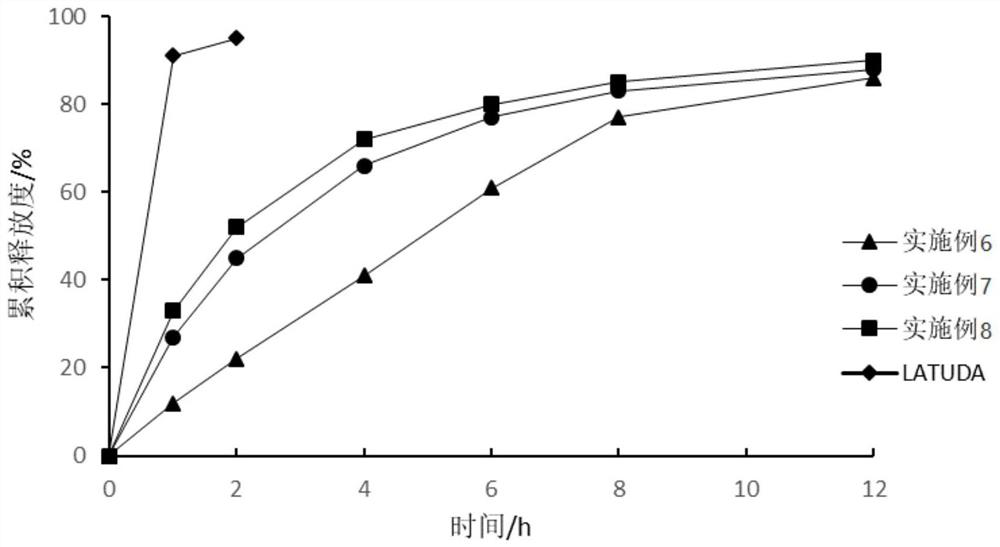
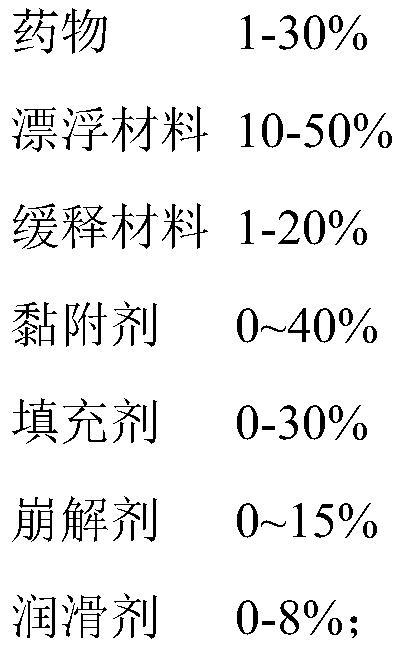
Sustained-release pharmaceutical composition and preparation method thereof
A technology for sustained-release medicines and compositions, applied in the field of sustained-release pharmaceutical compositions and their preparation, can solve the problems of loss of gastric floating effect and immediate floating of tablets, and achieve easy industrial scale-up production, good therapeutic effect and stable quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] prescription
[0142]
[0143] The preparation steps are:
[0144] Floating layer: fully mix polyvinyl acetate povidone mixture and polycarbomer;
[0145] Drug-containing layer: Lurasidone hydrochloride and hypromellose are granulated in a fluidized bed, and purified water is used as a wetting agent to prepare drug-containing layer granules;
[0146] The two-part material is compressed into a bilayer tablet.
Embodiment 2
[0148] prescription
[0149]
[0150] The preparation steps are:
[0151] floating layer:
[0152] 1) Fully mix polyvinyl acetate povidone mixture and polycarbomer;
[0153] 2) Add magnesium stearate to the above materials and mix evenly as a floating layer.
[0154] Drug-containing layer:
[0155] 1) Lurasidone hydrochloride and hypromellose are granulated in a fluidized bed, and purified water is used as a wetting agent to prepare drug-containing layer granules;
[0156] 2) Add magnesium stearate to the above granules and mix evenly to form a drug-containing layer.
[0157] The two-part material is compressed into a bilayer tablet.
Embodiment 3
[0159] prescription:
[0160]
[0161]
[0162] The preparation steps are:
[0163] floating layer:
[0164] 1) Fully mix polyvinyl acetate povidone mixture, carbomer, and cross-linked povidone;
[0165] 2) Add magnesium stearate to the above materials and mix evenly as a floating layer.
[0166] Drug-containing layer:
[0167] 3) Lurasidone hydrochloride and lactose hypromellose are granulated in a fluidized bed, and purified water is used as a wetting agent to prepare drug-containing layer granules;
[0168] 4) Add magnesium stearate to the above granules and mix evenly to form a drug-containing layer.
[0169] The two-part material is compressed into a bilayer tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com