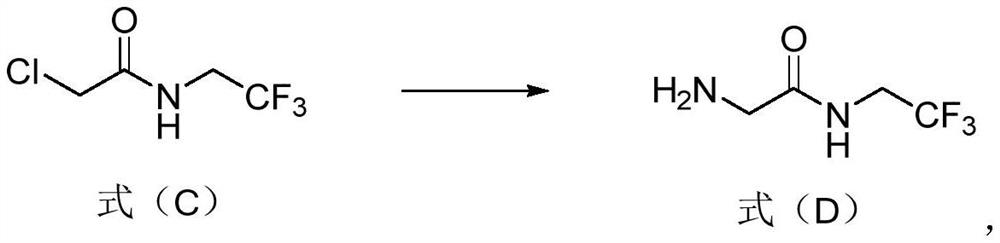
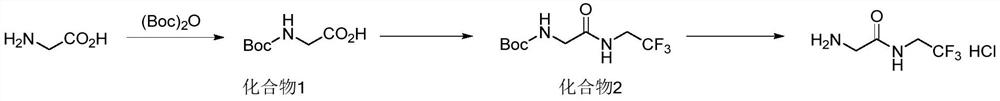
Preparation method of 2-amino-n-(2,2,2-trifluoroethyl)acetamide or salt thereof
A technology of trifluoroethylamine and chloroacetyl chloride is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc. The effect of industrialized production, low production cost and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of 2-chloro-N-(2,2,2-trifluoroethyl)acetamide
[0060] Add trifluoroethylamine hydrochloride (100.00g) and water (100.00g) into the reaction flask, stir and cool down in a low-temperature tank at -5°C; add precooled 30% aqueous sodium hydroxide solution (200.00g) and dichloro Methane (300.0g); add dropwise a dichloromethane solution of chloroacetyl chloride (86.62g of chloroacetyl chloride dissolved in 100g of dichloromethane) at an internal temperature of 0-5°C, and continue the reaction at 0-5°C for 1.5h ( Sampling and measuring GC, trifluoroethylamine <0.1% indicates that the reaction is complete); transfer to room temperature, stir and heat up to a temperature above 20°C in the bottle, stand for liquid separation; take the organic phase and evaporate to dryness under reduced pressure at 40°C to obtain 2- Chloro-N-(2,2,2-trifluoroethyl)acetamide: white solid 128.23g, yield 99.00%, GC detection, purity 99.33%.
Embodiment 2
[0061] Example 2: Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide hydrochloride
[0062] Add 2-chloro-N-(2,2,2-trifluoroethyl)acetamide (120.00g) and ammonia water (960.00g) into the reaction flask, react at 40°C for 2h, take 2 drops to measure GC (2-chloro- N-(2,2,2-trifluoroethyl)acetamide <0.1% indicates complete reaction); evaporated to dryness under reduced pressure at 60-70°C to obtain 132.79g of white solid (take a sample of 3mg, add 1mL of 1mol / L sodium carbonate aqueous solution Dissolve, extract with 1.5mL dichloromethane, take the lower layer solution and measure GC, the purity is 93.26%).
[0063] Add ethyl acetate (240.00 g) to the white solid obtained by evaporation to dryness above, and beat at room temperature for 2 h; filter, rinse the filter cake with 50 g of ethyl acetate, and dry it in a vacuum oven at 50 ° C for 8 h to obtain 2-amino-N-( 2,2,2-Trifluoroethyl)acetamide hydrochloride: 118.05 g of white solid, yield 89.68%, sampling by GC, purity 99...
Embodiment 3
[0065] Example 3: Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide hydrochloride
[0066] Add 2-chloro-N-(2,2,2-trifluoroethyl)acetamide (120.00g) and ammonia water (960.00g) into the reaction flask, react at 40°C for 2h, take 2 drops to measure GC (2-chloro- N-(2,2,2-trifluoroethyl)acetamide <0.1% indicates complete reaction); evaporated to dryness under reduced pressure at 60-70°C to obtain 135.13g of white solid (take a sample of 3mg, add 1mL of 1mol / L sodium carbonate aqueous solution Dissolve, extract with 1.5mL dichloromethane, take the lower layer solution and measure GC, the purity is 92.28%);
[0067] Add 240.00 g of 20% aqueous sodium hydroxide solution (W / W) to the white solid obtained by evaporation to dryness above. After dissolving, add 840.00 g of n-butanol, add solid sodium chloride until the water phase is saturated, and stir at room temperature for 0.5 h. Separation; add 420.00 g of n-butanol to the aqueous phase, extract, and combine the organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com