Propofol compound as well as preparation method and application thereof
A technology of propofol and compound, applied in the field of propofol compound and preparation thereof, can solve the problems of poor antioxidant performance, influence on clinical use effect, poor water solubility of propofol and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
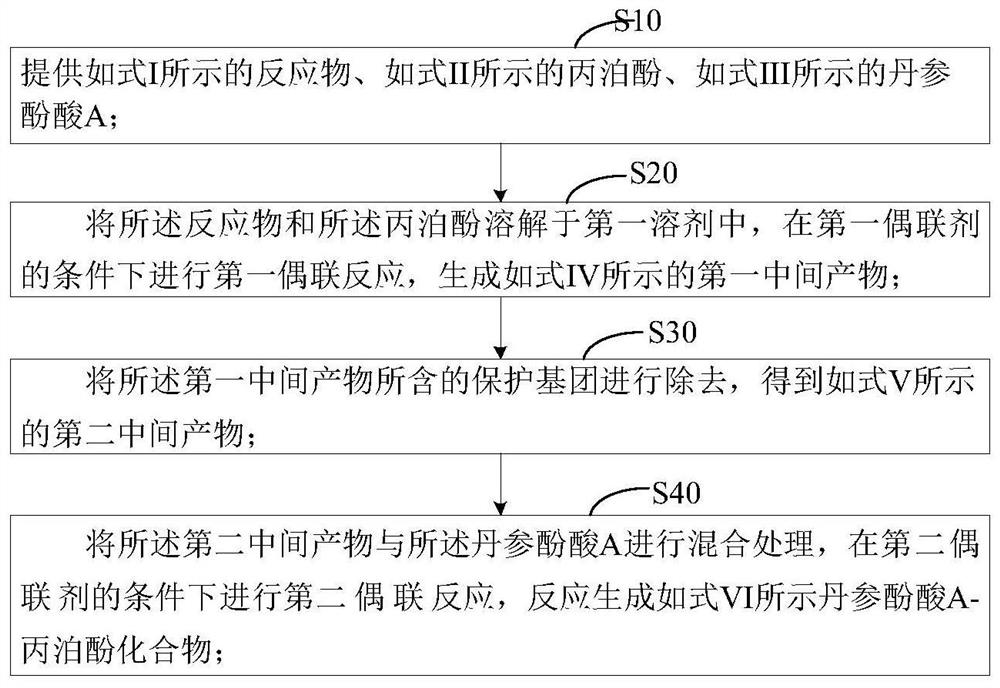
[0037] The second aspect of the embodiment of the present application provides a preparation method of a propofol compound, the preparation method comprising the following steps:
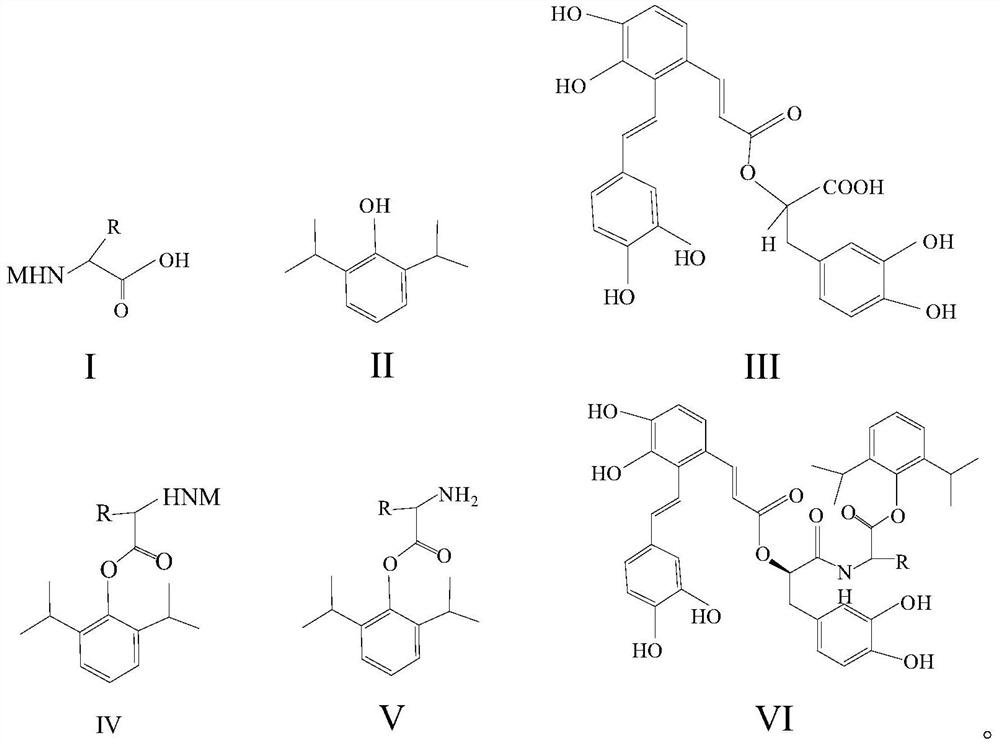
[0038] S10: providing the reactant shown in formula I, propofol shown in formula II, and salvianolic acid A shown in formula III;
[0039] S20: dissolving the reactant and propofol in a first solvent, and performing a first coupling reaction under the conditions of a first coupling agent to generate a first intermediate product as shown in formula IV;
[0040] S30: removing the protecting group contained in the first intermediate product to obtain a second intermediate product as shown in formula V;
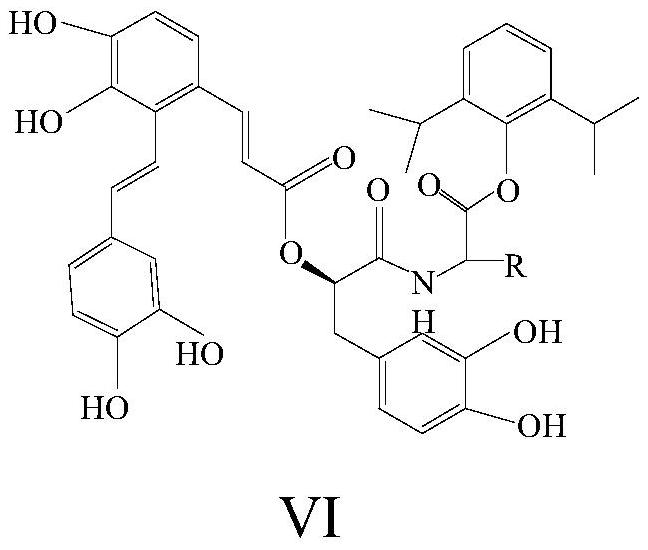
[0041] S40: Mix the second intermediate product with the salvianolic acid A, and perform a second coupling reaction under the conditions of the second coupling agent to generate salvianolic acid A-propofol as shown in formula VI compound;
[0042]Wherein, the R groups in the formulas I, IV, V and VI ...
Embodiment 1
[0068] The present embodiment provides a kind of preparation method of propofol compound, comprises the following steps:
[0069] S1: Provide 2-(tert-butylcarbonylamino)acetic acid, propofol, and salvianolic acid A with the R group being H as the reaction raw materials;
[0070] S2: 4.29g, 24.5mmol of 2-(tert-butylcarbonylamino)acetic acid and 3.3g, 18.5mmol of propofol were dissolved in dichloromethane, and 3.15g, 16.5mmol of propofol were added under nitrogen atmosphere EDC hydrochloride and 0.52g, 4.3mmol of DMAP, the resulting reaction mixture was stirred overnight at a temperature of 0-37°C, then the reaction mixture was washed with water twice, and dried with a desiccant containing magnesium sulfate; after solvent evaporation, Using hexane solvent column chromatography containing 1%-10% ethyl acetate to obtain yellow liquid 2,6-diisopropylphenyl 2-(tert-butylcarbonylamino) acetate (the first intermediate product) . Its reaction equation is as follows:
[0071]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com