Preparation method of chloramine
A chlorinated amine and hydroxylamine salt technology, applied in the field of organic synthesis, can solve the problems of long reaction period, high temperature, increased risk factor, etc., and achieve the effects of low cost, improved yield and purity, and increased safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
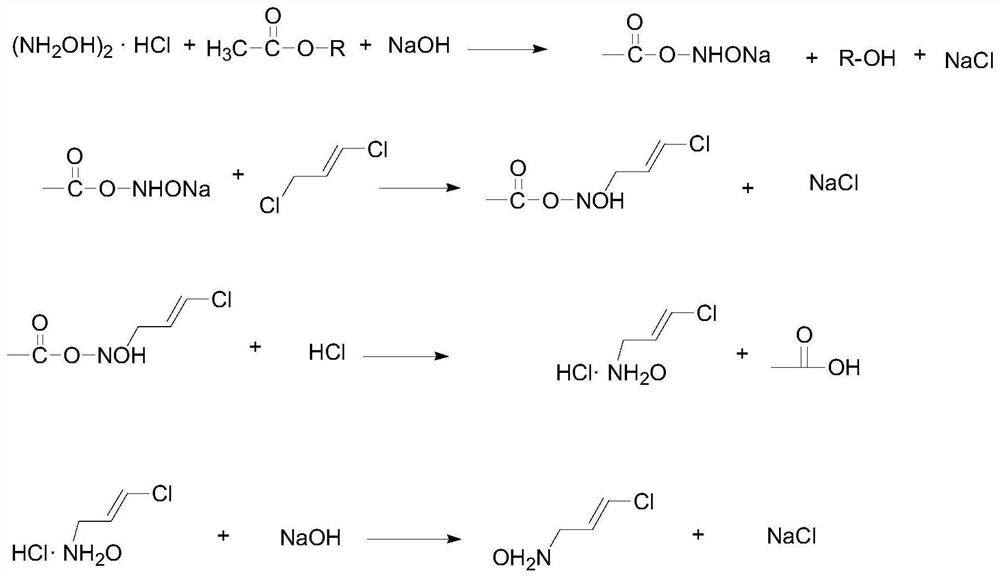
[0073] The preparation method of present embodiment chlorinated amine, comprises the following steps:
[0074] Hydroxylamine sulfate, acetate and 27% liquid caustic soda are entered in continuous flow reactor 1 with continuous feed pump respectively, and the feed rate of hydroxylamine salt is 15.92ml / min, and the feed rate of acetate is 7.7ml / min , the feed rate of liquid caustic soda is 7.8ml / min, the temperature of the integrated machine is controlled at 28°C, and the residence time is 164.05s to obtain the intermediate product A.
[0075] Intermediate product A is added 0.3% EDTA as catalyst to obtain mixture B, mixture B and 1,3-dichloropropene enter continuous flow reactor 2 at a certain flow rate, mixture B is 30ml / min, 1,3-dichloropropene The feed rate of the product was 5.88ml / min, the temperature of the integrated machine was controlled at 80°C, and the residence time was 164.05s to obtain the intermediate product C.
[0076] The intermediate product C and hydrochlor...
Embodiment 2
[0081] The preparation method of present embodiment chlorinated amine, comprises the following steps:
[0082] Hydroxylamine hydrochloride, acetate and 25% liquid caustic soda are entered in continuous flow reactor 1 respectively with continuous feed pump, and the feed rate of hydroxylamine salt is 15.92ml / min, and the feed rate of acetate is 7.7ml / min , the feed rate of liquid caustic soda is 7.8ml / min, the temperature of the integrated machine is controlled at 28°C, and the residence time is 164.05s to obtain the intermediate product A.
[0083] Intermediate product A is added 0.3% EDTA as catalyst, obtains mixture B, mixture B and 1,3-dichloropropene enter continuous flow reactor 2 at a certain flow rate, mixture B is 20ml / min, 1,3-dichloropropene The feed rate is 8.89ml / min, the temperature of the integrated machine is controlled at 80°C, and the residence time is 182.76s to obtain the intermediate product C.
[0084] The intermediate product C and hydrochloric acid are u...
Embodiment 3
[0089] The preparation method of present embodiment chlorinated amine, comprises the following steps:
[0090] Hydroxylamine sulfate, acetate and 30% liquid caustic soda are entered in continuous flow reactor 1 respectively with continuous feed pump, and the feed rate of hydroxylamine salt is 15.92ml / min, and the feed rate of acetate is 7.7ml / min , the feed rate of liquid caustic soda is 7.8ml / min, the temperature of the integrated machine is controlled at 28°C, and the residence time is 164.05s to obtain the intermediate product A.
[0091] Intermediate product A is added 0.3% EDTA as catalyst, obtains mixture B, mixture B and 1,3-dichloropropene enter continuous flow reactor 2 at a certain flow rate, mixture B is 20ml / min, 1,3-dichloropropene The feed rate was 8.89ml / min, the temperature of the integrated machine was controlled at 80°C, and the residence time was 182.76s to obtain the intermediate product C.
[0092] The intermediate product C and hydrochloric acid are used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com