Synthesis method of polyketone ligand
A synthesis method and ligand technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as handling difficulties and increase production costs, and achieve simple equipment and safe industry. Chemical, high product yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
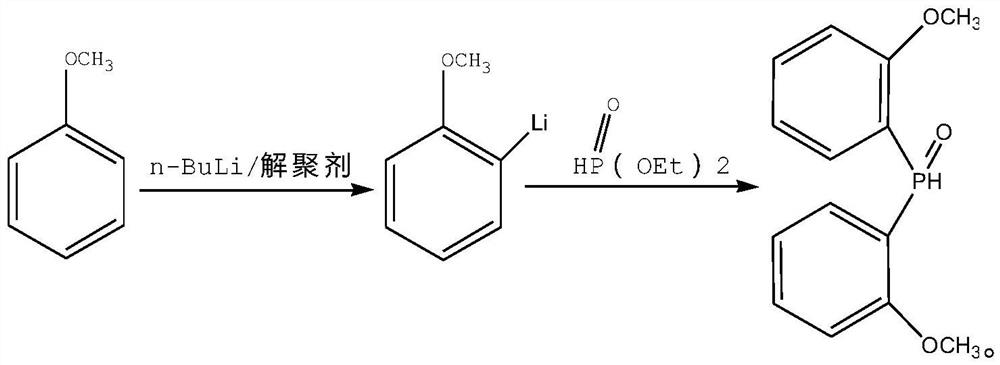
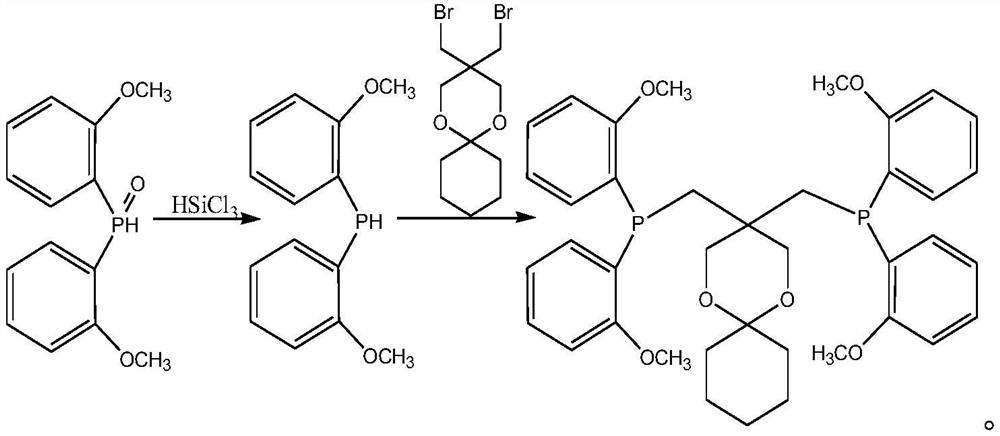
[0024] The present invention provides a polyketone ligand 3,3-dual-[double-(2-methoxyphenyl) phosphine methyl] -1,5-dioxane-snail [5, 5] extol Synthetic method, including the following steps:
[0025] a) Reaction the benzoatoxyl ether to the phosphite in the solvent to give a bis (2-methoxyphenyl) phosphine oxide;
[0026] b) The dual (2-methoxyphenyl) phosphine oxide obtained by step a) is reduced in a solvent to obtain a bis (2-methoxyphenyl) phosphine;
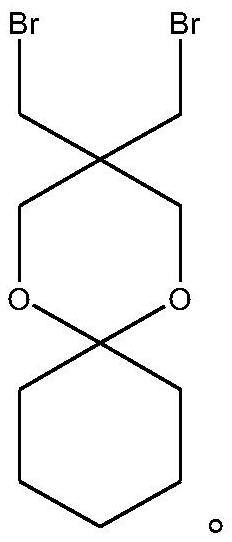
[0027] c) Different (2-methoxyphenyl) phosphine obtained by step b) under the action of the base, in the solvent and 5,5-bis (bromomethyl) -1,5-dioxane-snail [5, 5] Elementalk reaction to obtain polyketone ligand 3,3-dual-[double-(2-methoxyphenyl) phosphine methyl] -1,5-dioxane-snail [5, 5] Elementane.
[0028] The present invention first reacts benzoatoxyl ether to phosphite in a solvent to obtain a bis (2-methoxyphenyl) phosphine oxide. In the present invention, the solvent is preferably tetrahydrofuran and / or diethyl ether...
Embodiment 1
[0067] Method for synthesis of polyketone ligand 3,3-dual-[double-(2-methoxyphenyl) phosphine methyl] -1,5-dioxane [5,5] udeolicane, The specific steps are:
[0068] (1) Preparation of bis (2-methoxyphenyl) phosphine oxide:
[0069] Under anhydrous aerobic conditions, 2.70 g of the benzoyl ether was added to 2.90 ml of tetrahydrofuran, cooled to -20 ° C, start holding the temperature drop of 15.62 ml N-Buli, after the drop is added. Toning temperature was stirred for 1 h, warmed to 30 ° C for 4 h, followed by the tracking detection reaction, cooled to -20 ° C, start controlling the temperature drop of diaphragite 1.38 g, after the dropping, after 1 h, then heated to 30 ° C After the tracking detection reaction is completed, the reaction solution slowly dripped in ice water with 10% hydrochloric acid, and the pH is adjusted to be filled with water. To give a double (2-methoxyphenyl) phosphine oxide of 2.50 g, purity 98.65%, yield 94.13%.
[0070] (2) Preparation of bis (2-methoxyph...
Embodiment 2
[0075] Method for synthesis of polyketone ligand 3,3-dual-[double-(2-methoxyphenyl) phosphine methyl] -1,5-dioxane [5,5] udeolicane, The specific steps are:
[0076] (1) Preparation of bis (2-methoxyphenyl) phosphine oxide:
[0077] Under anhydrous aerobic conditions, the benzo ether 2.81 g and tetramethylenediamine was added to 20 ml of diethyl ether, cooled to -40 ° C, start holding the temperature drop plus 16.25 ml N-Buli solution, after the drop is added Toning temperature was stirred for 1 h, warmed to 40 ° C for 4 h, followed by the test reaction, cooled to -40 ° C, start controlling diethyl phosphite 1.38 g of phosphite, after the dropping, after 2 h, heated to 40 ° C After the tracking detection reaction is completed, the reaction solution slowly dripped in ice water with 10% hydrochloric acid, and the pH is adjusted to be filled with water. To give a double (2-methoxyphenyl) phosphine oxide of 2.55 g, purity 99.21%, yield 96.60%.
[0078] (2) Preparation of bis (2-methox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com