Synthesis method of levonorgestrel
A technology of levonorgestrel and its synthetic method, which is applied in the field of drug synthesis, can solve the problems of difficult complete reaction of raw materials, large amount of tetrahydrofuran, and influence on the reaction process, etc., and achieves simplified production process, reduced separation and purification operations, and good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] One embodiment of the present invention provides a synthesis method of levonorgestrel, comprising the following steps S10-S30.
[0034] Step S10: alkynylation of the compound of formula (I) and trialkylsilylacetylene in the first organic solvent under the action of an organometallic reagent to obtain a reaction solution containing the compound of formula (II);
[0035]
[0036] Wherein, the chemical name of the compound of formula (I) is 18-methylestro-2,5(10)-diene-3-methoxyl-17-ketone, also known as ethyl Woshi; formula (II ) compound has the chemical name 13β-ethyl-18,19-bisanor-17β-hydroxypregna-2,5(10)-diene-3-methoxy-20-trialkylalkynyl.
[0037] In some examples, in step S10, the organometallic reagent is n-butyllithium or lithium diisopropylamide. Alternatively, the organometallic reagent is n-butyllithium.
[0038] In some examples, in step S10, the alkyl group of the trialkylsilylacetylene has 1-5 carbon atoms. Optionally, the alkyl group of the trialkyls...
Embodiment 1
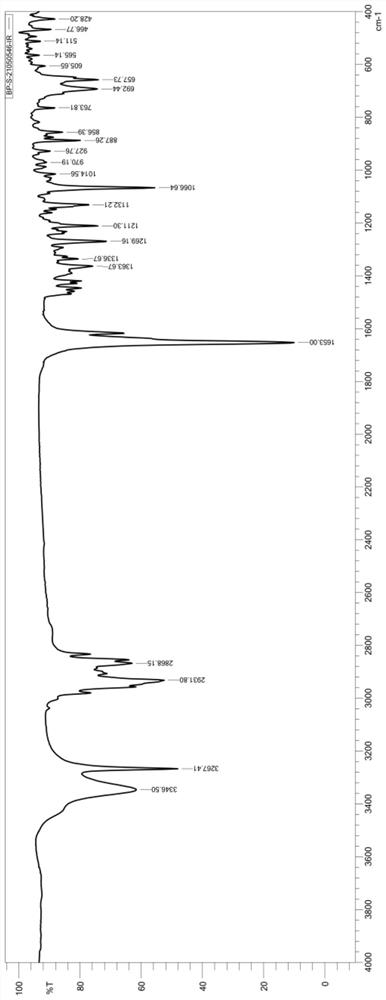
[0078] Add 500 mL of THF to 450 mL of n-butyllithium (2.5 M) solution, cool to -10°C, add 105 g of trimethylsilylacetylene dropwise, and keep warm at -10 to 0°C for 0.5 hours. Add 100 g of ethyl Worcester, and keep the reaction at -10~0°C for 2 hours. After adding 400 mL of methanol, 100 mL of water was added, and the temperature was raised to 20° C. to 30° C. to react for 2 hours. 200 g of concentrated hydrochloric acid was added, and the temperature was raised to 50° C. to 60° C. to react for 2 hours. Add potassium carbonate solution for neutralization, concentrate to a certain amount, add 500 mL of water for water analysis, and filter to obtain crude levonorgestrel. The crude product of levonorgestrel was dissolved by adding acetone to raise the temperature, cooled and crystallized, filtered, and then refined once with acetone to obtain 93.6 g of white solid with an HPLC purity of 99.2%. After testing, the infrared spectrum of the white solid is as follows figure 1 Shown...
Embodiment 2
[0080] Add 600 mL of n-butyllithium (2.5M) solution to dilute with 600 mL of ethylene glycol dimethyl ether, cool to -20°C, add 155 g of trimethylsilylacetylene dropwise, and keep warm at -15 to 0°C for 0.5 hours. Add 100 g of ethyl Worcester, keep warm at -15 to 0°C and react for 3 hours. After adding 400 mL of methanol, 100 mL of water was added, and the temperature was raised to 20° C. to 30° C. to react for 2 hours. Add 250 g of hydrobromic acid, raise the temperature to 60-70°C and react for 1 hour. Add potassium hydroxide solution for neutralization, concentrate to a certain amount, add 500 mL of water for water analysis, and filter to obtain a crude product. The crude product was dissolved in tetrahydrofuran and then evaporated to a small amount to obtain a white solid. It was refined once more with acetone to obtain 86.3 g of levonorgestrel with an HPLC purity of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com