Triarylboron conjugated polymer porous material as well as preparation method and application thereof
A technology of conjugated polymer and triaryl boron, which is applied in the field of triaryl boron conjugated polymer porous materials and its preparation, can solve the problems of uneconomical preparation methods and application limitations, and achieve good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A kind of triaryl boron conjugated polymer porous material P 1 The synthetic route and steps of -Th are as follows:
[0059]
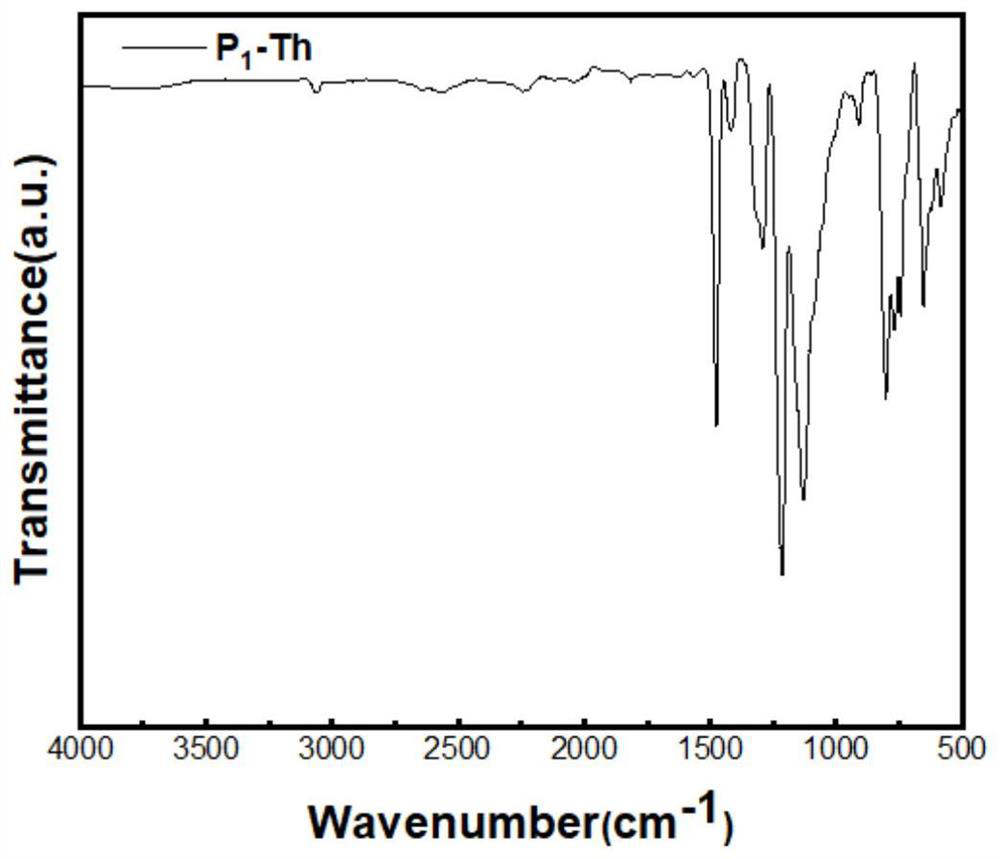
[0060] Join BBr first 3 (0.424g, 1.69mmol) in a 100-mL Schlenk tube, followed by 10mL of dichlorobenzene, the compound 2,5-bis(trimethyltin-based)thiophene (1.03g, 2.51mmol) was dissolved in dichloro Benzene was added dropwise slowly. At this time, a large number of yellow precipitates were formed, and the stirring was increased, and the total amount of solvent was added to 30 mL. The Schlenk tube was transferred to an oil bath, and the branch port was protected with nitrogen at 180 ° C for 3 days. After the reaction time is over, transfer the Schlenk tube to the glove box after cooling. The solid was repeatedly washed with dichloromethane in a glove box and filtered through a sand core funnel. Finally, the solid was transferred to a vial and extracted under vacuum for 4 hours to obtain 240.0 mg of a yellow solid. 11 B MAS SSNMR(400MHz)δ...
Embodiment 2
[0062] A kind of triaryl boron conjugated polymer porous material P 2 The synthetic route and steps of -Th are as follows:
[0063]
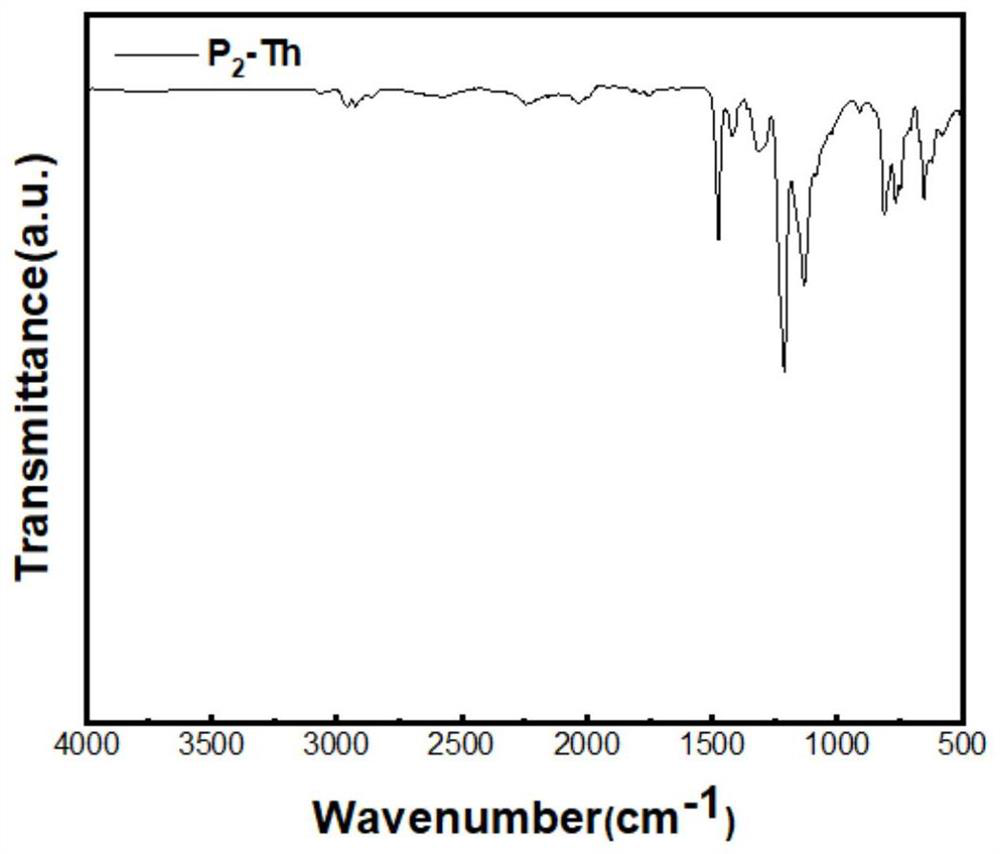
[0064] Compound P 2 The synthetic method of -Th is the same as that of compound P 1 -Th (Example 1). Feed: BBr 3 (0.371g, 1.48mmol), compound 2,5-bis(tri-n-butyltinyl)thiophene (1.47g, 2.22mmol), finally obtained 204.0mg of yellow solid. 11 B MAS SSNMR(400MHz)δ (iso) 46.1ppm; BET area: 648.7 m 2 / g; its infrared spectrum is as figure 2 shown.
Embodiment 3
[0066] A kind of triaryl boron conjugated polymer porous material P 1 -Th 2 The synthetic route and steps are as follows:
[0067]
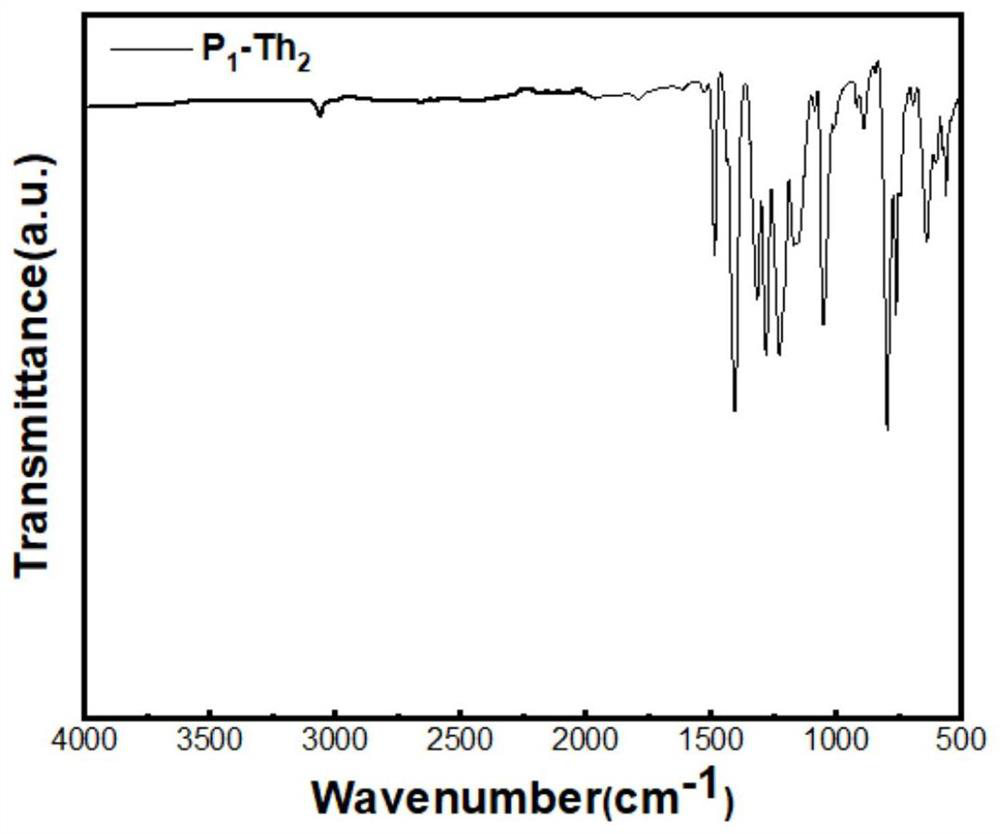
[0068] Compound P 1 -Th 2 The synthetic method is with embodiment 1. Feed: BBr 3 (0.374g, 1.49mmol), the compound 5,5'-bis(trimethyltin-yl)-2,2'-bithiophene (1.01g, 2.05mmol), finally obtained 369.6 mg of orange-red solid. 11 BMAS SSNMR(400MHz)δ (iso) 43.6ppm; BET area: 164.6m 2 / g; its infrared spectrum is as follows image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com