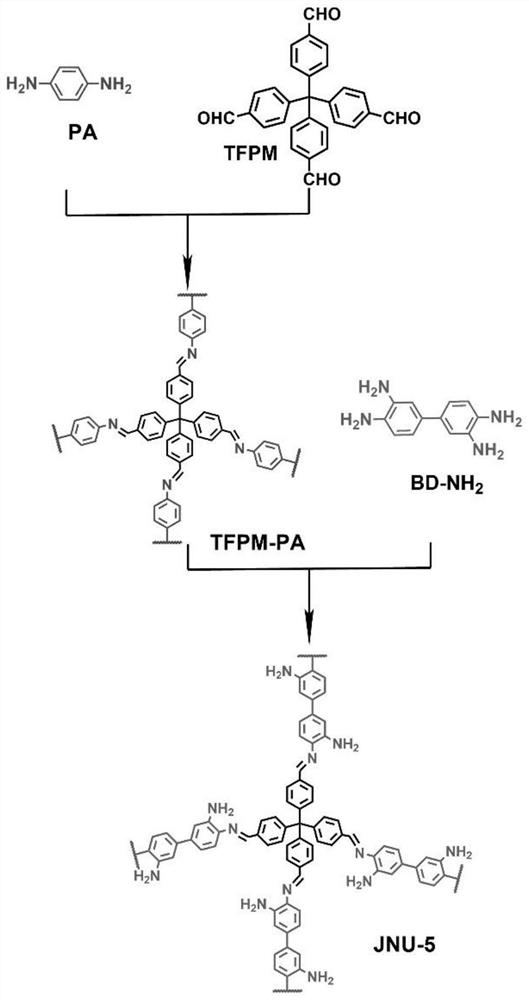
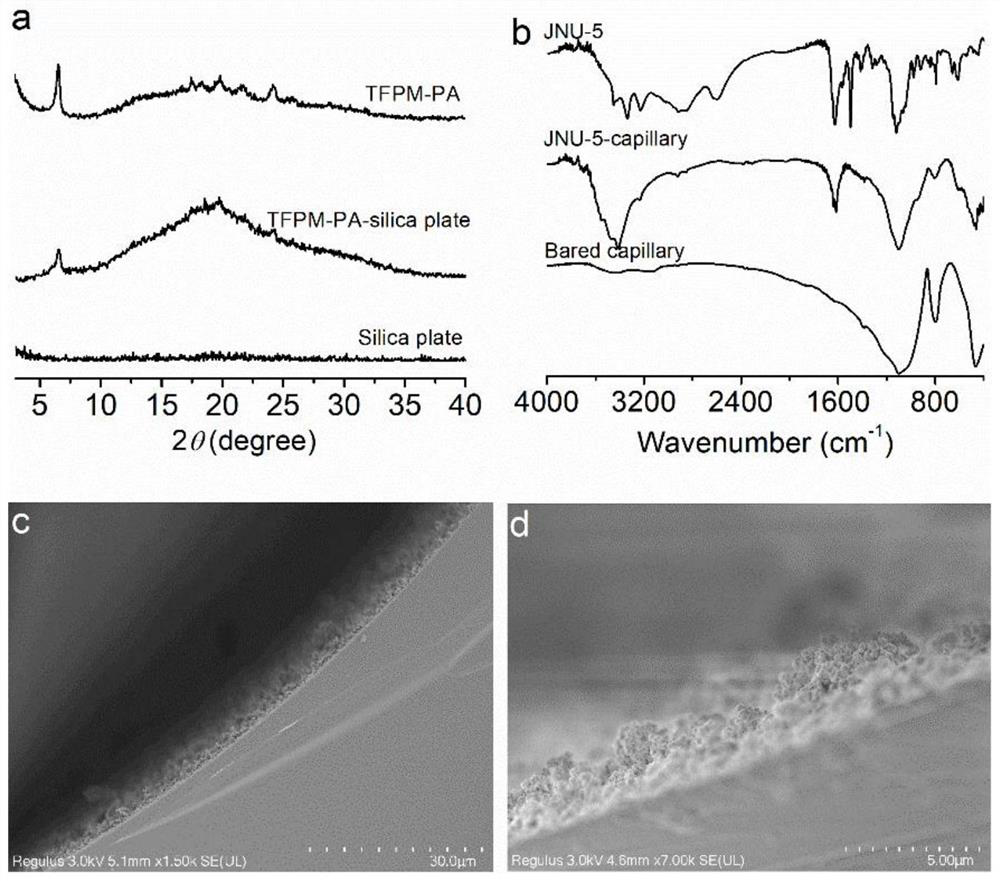
Bonded three-dimensional covalent organic framework chromatographic column and separation application thereof
A technology of covalent organic framework and chromatographic column, which is applied in the field of bonding three-dimensional covalent organic framework chromatographic column and its separation application, can solve the problem that the application of gas chromatography stationary phase has not yet been reported, and achieve high resolution and high column efficiency separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of a three-dimensional covalent organic framework-based capillary gas chromatography column: a bonded JNU-5 capillary column was prepared by an in-situ growth method, which specifically included the following steps:
[0038] (1) Fused silica capillary (10m long × 0.53mm inner diameter) was treated with 1M sodium hydroxide (NaOH) for 2h, 0.1M hydrochloric acid (HCl) for 2h, water until the effluent pH=7.0, and then treated with methanol for 30min.
[0039] (2) Then fill the methanol solution (50%, v / v) of 3-aminopropyltrimethoxysilane (APTES) into the capillary, react overnight at 40°C, rinse with methanol, and use nitrogen flow at 120°C After drying for 2 hours, APTES-modified capillaries were prepared.
[0040] (3) An ethanol solution of tetrakis(4-formylphenyl)-methane (TFPM) (21.6 mg of TFPM dissolved in 3 mL of ethanol) was further filled and reacted at 60° C. for 2 hours. Finally, the capillary column was rinsed with methanol to wash away the residue, ...
Embodiment 2
[0048] Preparation of a three-dimensional covalent organic framework-based capillary gas chromatography column:
[0049] (1) Fused silica capillary (1m, 100m long × 0.53mm inner diameter) was treated with 1M sodium hydroxide (NaOH) for 2h, 0.1M hydrochloric acid (HCl) for 2h, water treatment until the pH of the effluent = 7.0, and then treated with methanol 30min.
[0050] (2) Then fill the methanol solution (50%, v / v) of 3-aminopropyltrimethoxysilane (APTES) into the capillary, react overnight at 40°C, rinse with methanol, and use nitrogen flow at 120°C After drying for 2 hours, APTES-modified capillaries were prepared.
[0051] (3) An ethanol solution of tetrakis(4-formylphenyl)-methane (TFPM) (21.6 mg of TFPM dissolved in 3 mL of ethanol) was further filled and reacted at 60° C. for 2 hours. Finally, the capillary column was flushed with methanol to wash away the residue, and dried at 120 °C with a nitrogen stream for 2 h to obtain TFPM-modified capillaries.
[0052] (4)...
Embodiment 3
[0056] Preparation of a three-dimensional covalent organic framework-based capillary gas chromatography column:
[0057] (1) Fused silica capillary (10m long × 0.075, 0.53mm inner diameter) was treated with 1M sodium hydroxide (NaOH) for 2h, 0.1M hydrochloric acid (HCl) for 2h, water treatment until the effluent pH = 7.0, and then treated with methanol 30min.
[0058] (2) Then fill the methanol solution (50%, v / v) of 3-aminopropyltrimethoxysilane (APTES) into the capillary, react overnight at 40°C, rinse with methanol, and use nitrogen flow at 120°C After drying for 2 hours, APTES-modified capillaries were prepared.
[0059] (3) An ethanol solution of tetrakis(4-formylphenyl)-methane (TFPM) (21.6 mg of TFPM dissolved in 3 mL of ethanol) was further filled and reacted at 60° C. for 2 hours. Finally, the capillary column was flushed with methanol to wash away the residue, and dried at 120 °C with a nitrogen stream for 2 h to obtain TFPM-modified capillaries.
[0060] (4) TFPM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com