Preparation method and application of alpha-acetyl-gamma-butyrolactone
A technology of butyrolactone and acetyl group, which is applied in the field of preparation of α-acetyl-γ-butyrolactone, can solve the problems of low acylation reaction yield, large rectification loss, low purity and the like, and achieves improved acylation The effect of reaction yield, elimination of inhibition, and elimination of potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] According to a first aspect of the present invention, a method for preparing α-acetyl-γ-butyrolactone is provided, comprising the following steps:
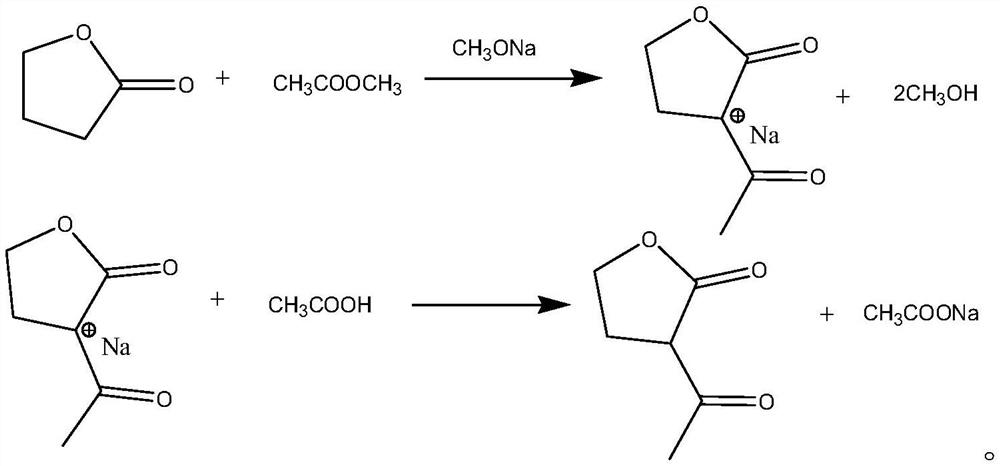
[0037] γ-butyrolactone, acylating reagents, alkaline reagents and benzene reagents are mixed for acylation reaction. At the same time of acylation reaction, acylating reagents, benzene reagents and by-products are azeotroped to remove by-products. And obtain α-acetyl-γ-butyrolactone after;
[0038] Wherein, the alkaline reagent includes an alkoxide solution, and the by-product is a by-product generated by the alkaline reagent.
[0039] In the present invention, the alkoxide solution is used instead of the traditional solid alkoxide as the alkaline reagent for the acylation of γ-butyrolactone, thus eliminating the potential safety hazards caused by the use of solid alkaline reagents and enabling closed material transportation and automatic reaction control; and the present invention introduces benzene-based reagents into th...
Embodiment 1
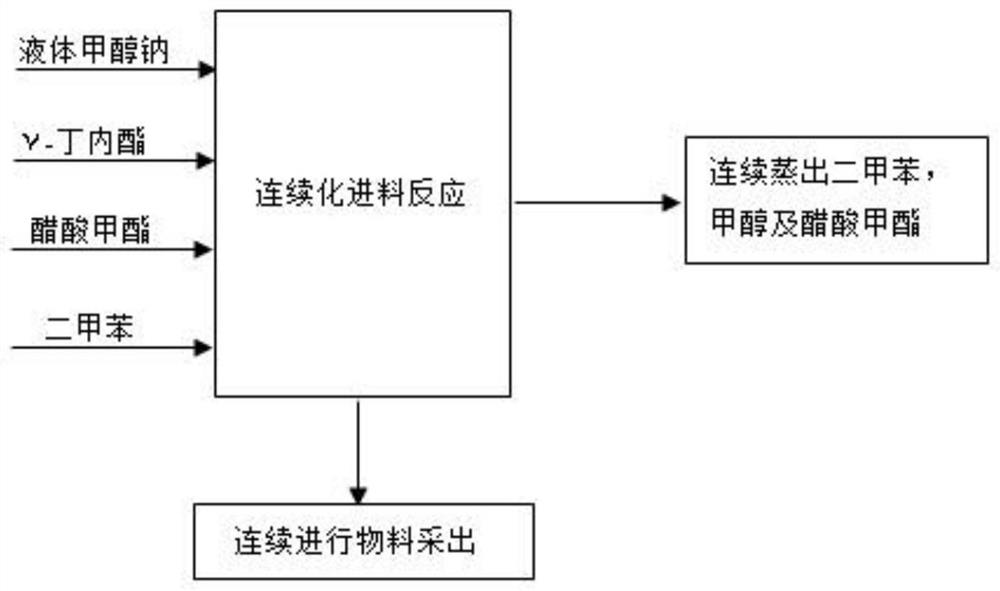
[0060] The preparation method of α-acetyl-γ-butyrolactone, the flow process is as follows figure 1 , including the following steps:
[0061] Continuously press into γ-butyrolactone 5g / min, methyl acetate 12.5g / min, 29% liquid sodium methylate 14.65g / min and xylene 3g / min in the reaction equipment, while reacting solvent distillation, utilize xylene, Methyl acetate and the methyl alcohol three that produce are azeotropic, and the methyl alcohol distillation that suppresses reaction is carried out (its collecting speed is 22.5g / min, and meteorological detection methanol content is 62.2%, xylene content is 12.9%, and methyl acetate content is 24.9%);
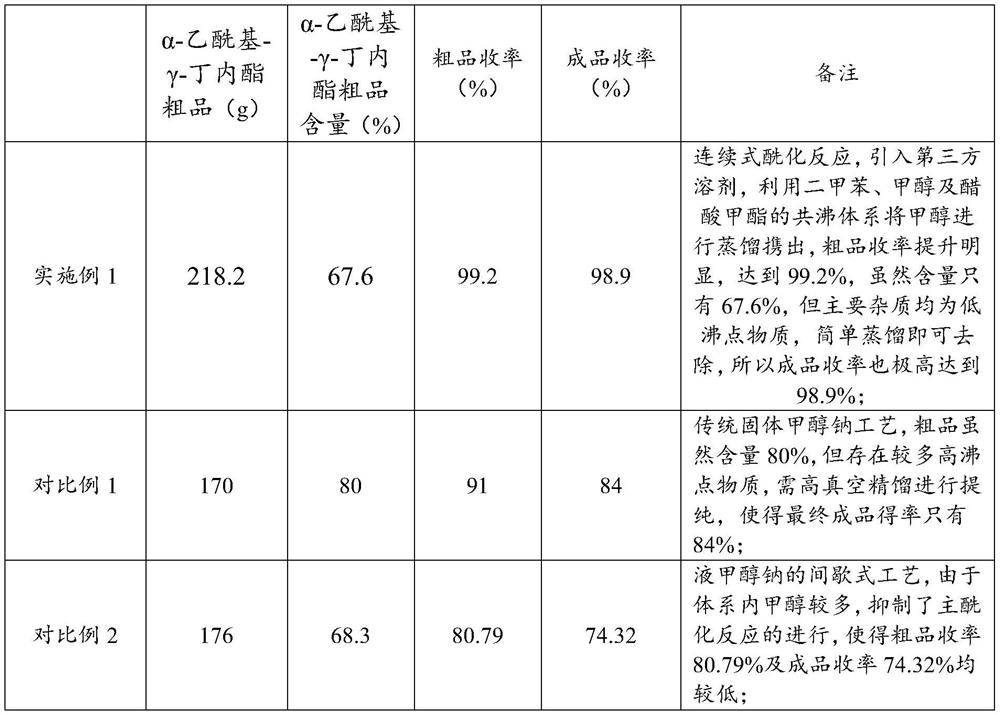
[0062] After the above-mentioned continuous feeding for 10 minutes, the reaction product began to be discharged while feeding. After 20 minutes, a total of 253g of materials were collected, and after cooling, they were neutralized with 95g of anhydrous acetic acid to obtain sodium acetate and α-acetyl-γ-butyrolactone Crude produc...
Embodiment 2
[0066] The preparation method of α-acetyl-γ-butyrolactone comprises the following steps:
[0067] Continuously press 5 g / min of gamma-butyrolactone, 12.5 g / min of methyl acetate, 19 g / min of 29% liquid potassium methylate and 3 g / min of xylene in the reaction equipment, distill solvent while reacting, utilize xylene, acetic acid Methyl ester and the methyl alcohol three that produce azeotrope, the methyl alcohol distillation that suppresses reaction is carried out (its collecting speed is 22.5g / min, and meteorological detection methyl alcohol content is 62.2%, xylene content is 12.9%, and methyl acetate content is 24.9% %); ·
[0068] After the above-mentioned continuous feeding for 10 minutes, the reaction product began to be discharged while feeding. After 20 minutes, a total of 340 g of materials were collected, and after cooling, they were neutralized with 95 g of anhydrous acetic acid to obtain sodium acetate and α-acetyl-γ-butyrolactone Crude product, after separation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com