Characterization method of pharmaceutical co-crystal performance
A drug and performance technology, applied in the field of characterization of drug co-crystal properties, can solve problems such as low solubility, sample destructiveness, slow oral absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
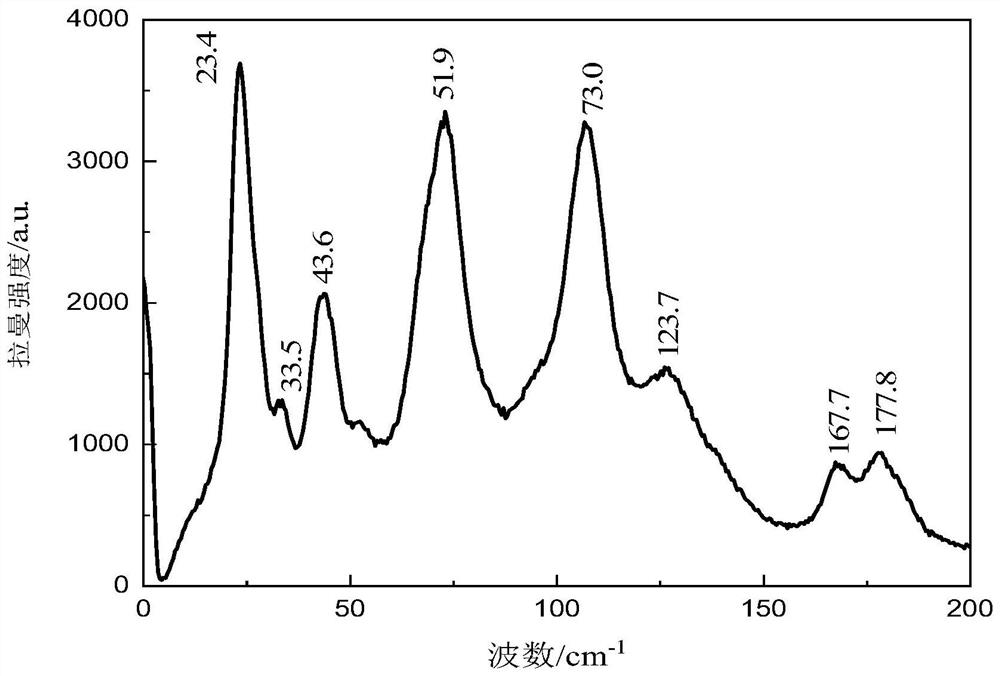
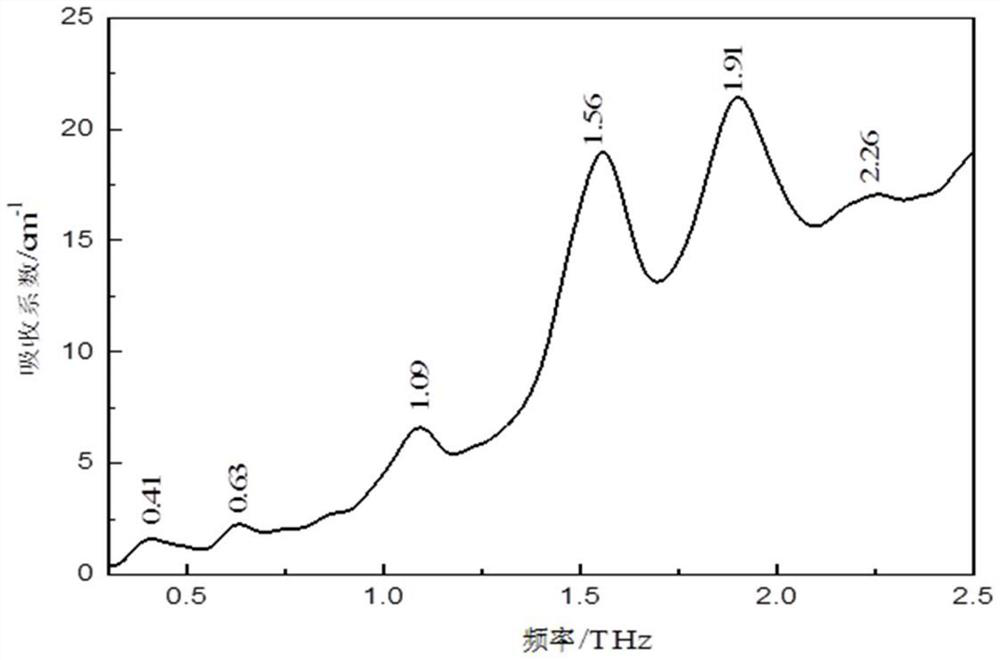
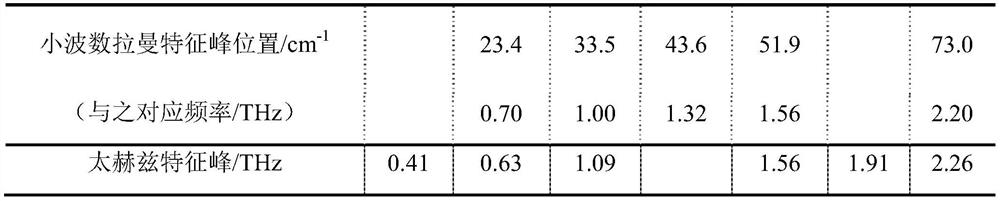
[0026] The present invention will be described in further detail below in conjunction with the accompanying drawings and specific embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0027] The present invention proposes a method for characterizing low-frequency vibrations of drug eutectics, based on terahertz spectroscopy and Raman spectroscopy, including the following steps:
[0028] 1. Sample preparation:
[0029] (101) Preparation of drug co-crystal sample A: use solution evaporation method to prepare drug co-crystal sample A with a molar ratio of 1:1; drug co-crystal sample A in this embodiment is carbamazepine-nicotinamide drug co-crystal; specific : Weigh carbamazepine and nicotinamide with a molar ratio of 1:1, dissolve them in an appropriate amount of absolute ethanol, stir at room temperature for 3 hours, then slowly volatilize at room temperature, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com