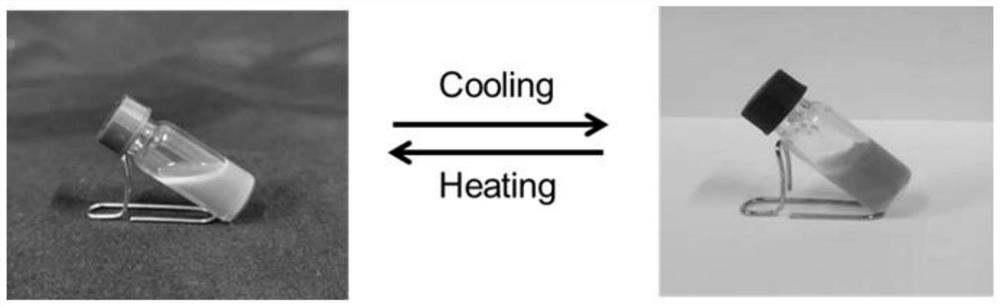
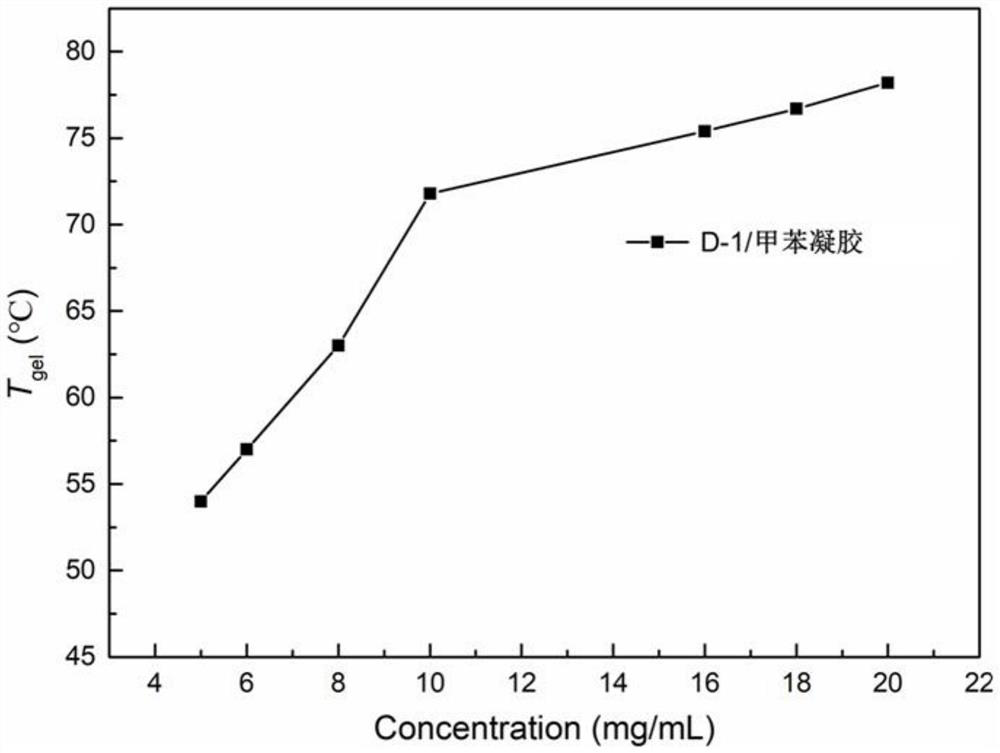
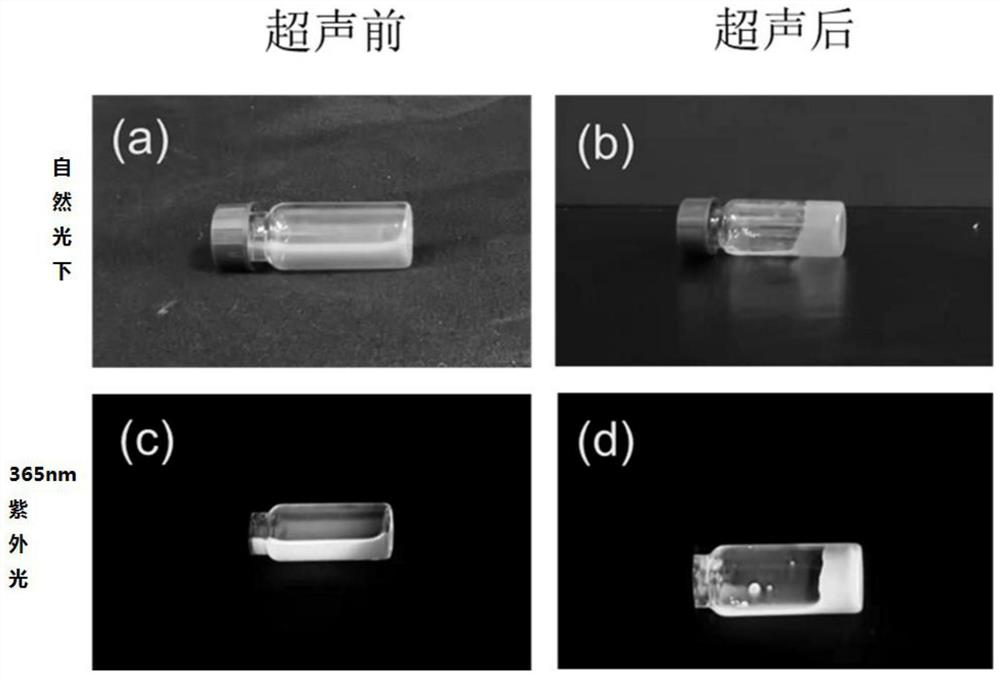
Quinacridone cholesterol compound as well as preparation method and application thereof
A technology for quinacridone cholesterol and compounds, applied in the field of quinacridone cholesterol compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] Quinolino[2,3-b]-acridine-5,12-dihydro-7,14-dione (3.12g, 10mmol), 1,6-dibromohexane (12.20g, 50mmol), Sodium hydride (1.20g, 50mmol) and the solvent THF 50mL were placed in a 150mL pressure bottle and mixed, heated and stirred under the protection of nitrogen for reflux reaction for 24h, and the solid was removed by suction filtration with a suction filter flask, and the obtained filtrate was rotary evaporated to remove the solvent to obtain crude The product was chromatographed on a silica gel column with dichloromethane:petroleum ether=1:1 as a developing solvent to obtain 4.15g of solid compound A-1 with a yield of 65%.
[0055]
[0056] Compound A-1 (3.19g, 5mmol), phthalimide potassium salt (9.26g, 50mmol) and 50mL N,N-dimethylformamide were placed in a 250mL reaction flask and mixed under argon protection Heat and stir at 60°C for 24 hours, add a large amount of water to the reaction solution to precipitate a solid, filter and dry, and purify the c...
Embodiment 2
[0064]
[0065] The synthesis steps of compound A-2 are similar to those of compound A-1 in Example 1, except that the 1,6-dibromohexane used in Example 1 is replaced by 1,8-dibromooctane (13.60g, 50mmol), the synthesis steps of the remaining compounds are the same as in Example 1, and the amount of each compound is also the same as in Example 1.
[0066]
[0067] The preparation of compound B-2 was carried out with reference to Example 1, and the dosage of each compound was also the same as that in Example 1.
[0068]
[0069] The preparation of compound C-2 was carried out with reference to Example 1, and the dosage of each compound was also the same as that in Example 1.
[0070]
[0071] The preparation of compound D-2 was carried out with reference to Example 1, and the amount of each compound was also the same as that in Example 1. The relevant data of compound D-2 are as follows: (QA-C 8 -Chol, 55%): 1 H NMR (400MHz, CDCl 3): δ8.66(s, 2H, QA), 8.51(dd, J...
Embodiment 3
[0073]
[0074] The synthesis steps of compound A-3 are similar to those of compound A-1 in Example 1, except that the 1,6-dibromohexane used in Example 1 is replaced by 1,10-dibromodecane (15.00g, 50mmol), the synthesis steps of the remaining compounds are the same as in Example 1, and the amount of each compound is also the same as in Example 1.
[0075]
[0076] The preparation of compound B-3 was carried out with reference to Example 1, and the dosage of each compound was also the same as that in Example 1.
[0077]
[0078] The preparation of compound C-3 was carried out with reference to Example 1, and the dosage of each compound was also the same as that in Example 1.
[0079]
[0080] The preparation of compound D-3 was carried out with reference to Example 1, and the dosage of each compound was also the same as that in Example 1. The relevant data of compound D-3 are as follows: (QA-C 10 -Chol, 58%): 1 H NMR: (400MHz, CDCl 3 ): δ8.71(s, 2H, QA), 8.53(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com