Self-flashing fluorescent dye for long-time super-resolution fluorescence imaging of lysosome as well as synthesis method and application of self-flashing fluorescent dye
A technology of super-resolution fluorescence and fluorescent dyes, applied in the direction of fluorescence/phosphorescence, azo dyes, organic dyes, etc., can solve the problem of difficult to realize the transition to dark state, and achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of Dye Lyso-532
[0034]
[0035] Rho19 (100mg, 0.22mmol) was placed in 20mL of 1,2-dichloroethane, then 1.0mL of phosphorus oxychloride was added to the reaction solution, and reacted at 80°C for 4h. The reaction solution was cooled and removed under reduced pressure to obtain a purple crude product. The crude product was dissolved in 20 mL of acetonitrile and 0.5 mL of triethylamine was added followed by 2-amino-6-picoline (400 mg, 2.16 mmol). Reaction at 80°C for 10h. The solvent was removed under reduced pressure, and basic alumina column chromatography (developing solvent: dichloromethane:methanol=400:1; volume ratio) gave 50 mg of white solid with a yield of 45%.
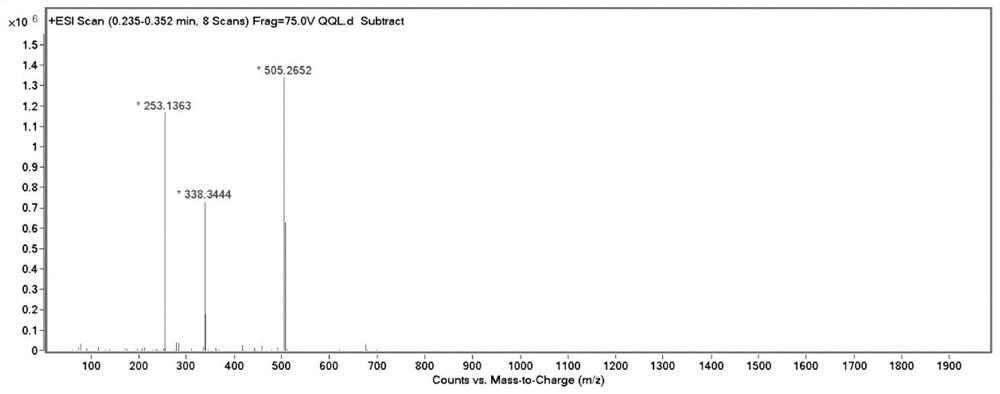
[0036] Its high-resolution mass spectrometry data are as follows:
[0037] HRMS(ESI)m / z[M+H] + : Calculated: 505.2604, Experimented: 505.2652.
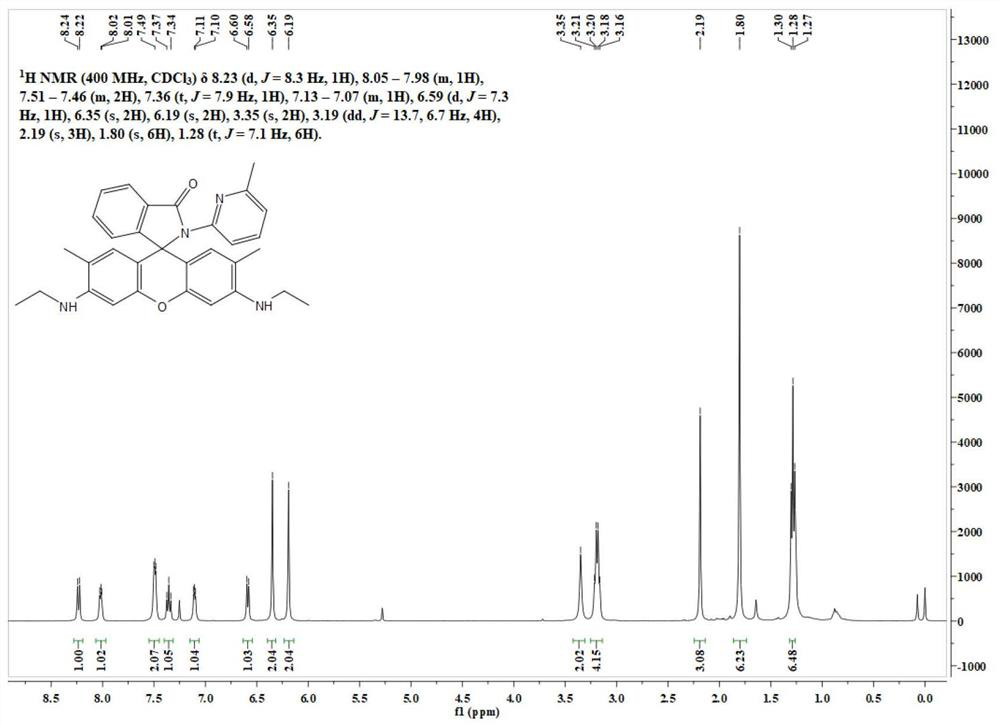
[0038] Its NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.23(d, J=8...
Embodiment 2
[0046] Synthesis of Dye LysoNH-532
[0047]
[0048] Rho19 (300mg, 0.67mmol) was placed in 30mL of 1,2-dichloroethane, and then 2.0mL of phosphorus oxychloride was added to the reaction solution, and reacted at 80°C for 8h. The reaction solution was cooled and removed under reduced pressure to obtain a purple crude product. The crude product was dissolved in 30 mL of acetonitrile and 0.5 mL of triethylamine was added followed by 2-amino-5-methylaminocarbonylpyridine (900 mg, 5.95 mmol). Reaction at 80°C for 24h. The solvent was removed under reduced pressure, and basic alumina column chromatography (developing solvent: dichloromethane:methanol=300:1; volume ratio) gave 93 mg of white solid with a yield of 25%.
[0049] Its high-resolution mass spectrometry data are as follows:
[0050] HRMS(ESI)m / z[M+H] + : Calculated value: 548.2662, Experimental value: 548.2671.
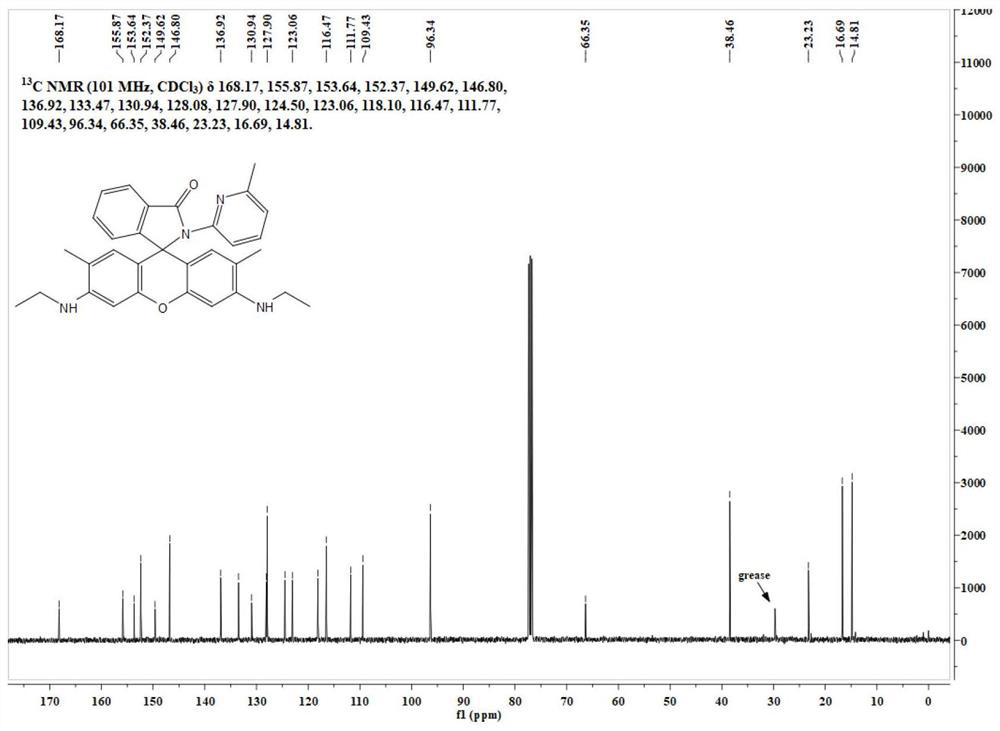
[0051] The specific data of its NMR spectrum are as follows:
[0052] 1 H NMR (400MHz, CDCl 3 )δ8.22(...
Embodiment 3
[0055] Synthesis of Dye LysoH-532
[0056]
[0057] Rho19 (100mg, 0.22mmol) was placed in 15mL of 1,2-dichloroethane, then 0.5mL of phosphorus oxychloride was added to the reaction solution, and reacted at 80°C for 4h. The reaction solution was cooled and removed under reduced pressure to obtain a purple crude product. The crude product was dissolved in 15 mL of acetonitrile and 0.5 mL of triethylamine was added followed by 2-aminopyridine (500 mg, 5.32 mmol). Reaction at 80°C for 12h. The solvent was removed under reduced pressure, and basic alumina column chromatography (developing solvent: dichloromethane:methanol=400:1; volume ratio) gave 74 mg of a white solid with a yield of 67%.
[0058] Its high-resolution mass spectrometry data are as follows:
[0059] HRMS(ESI)m / z[M+H] + : Calculated value: 491.2447, Experimental value: 491.2442.
[0060] The specific data of its NMR spectrum are as follows:
[0061] 1 H NMR (400MHz, CDCl 3 )δ8.23(d,J=8.1Hz,1H),8.04–8.00(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com