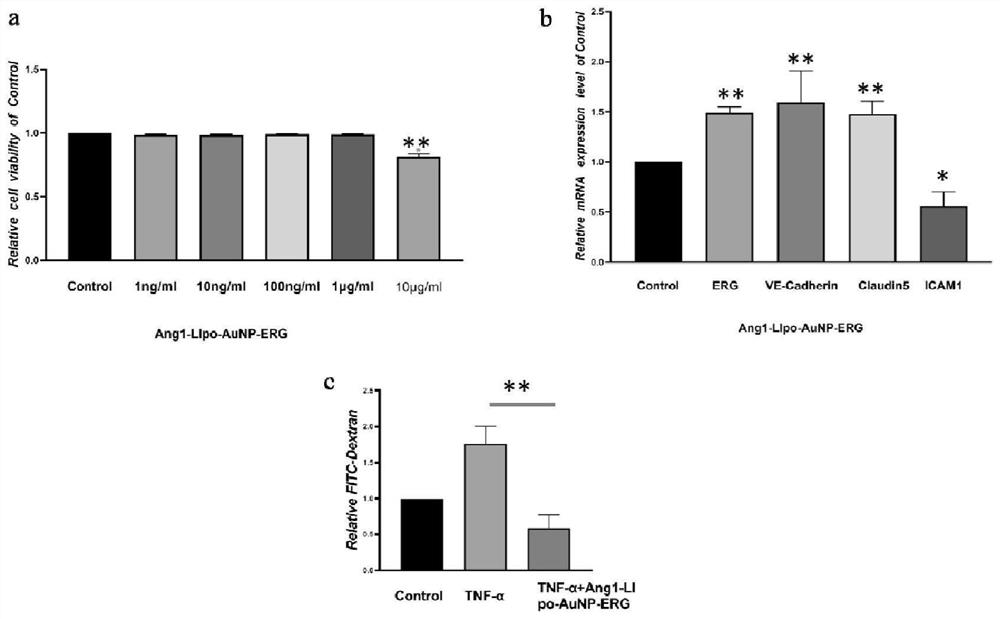
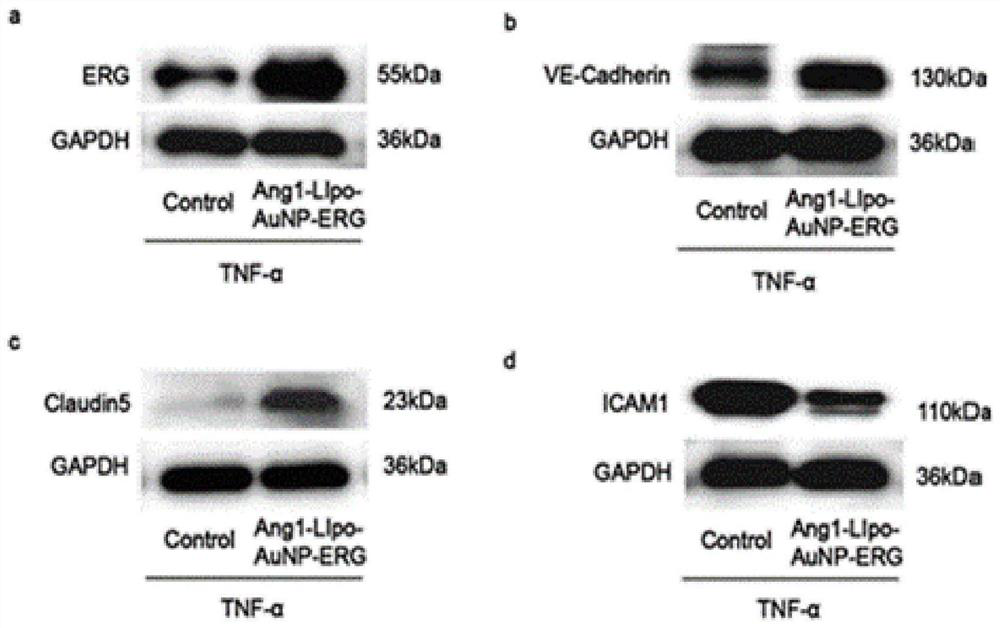
Lipid nanogold particle compound and application thereof in delivering ERG and treating encephaledema disease
A technology of gold nanoparticles and composites, applied in gene therapy, genetic material components, pharmaceutical formulations, etc., can solve the problem of less brain edema, achieve the effects of treating brain edema, repairing the blood-brain barrier, and facilitating large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
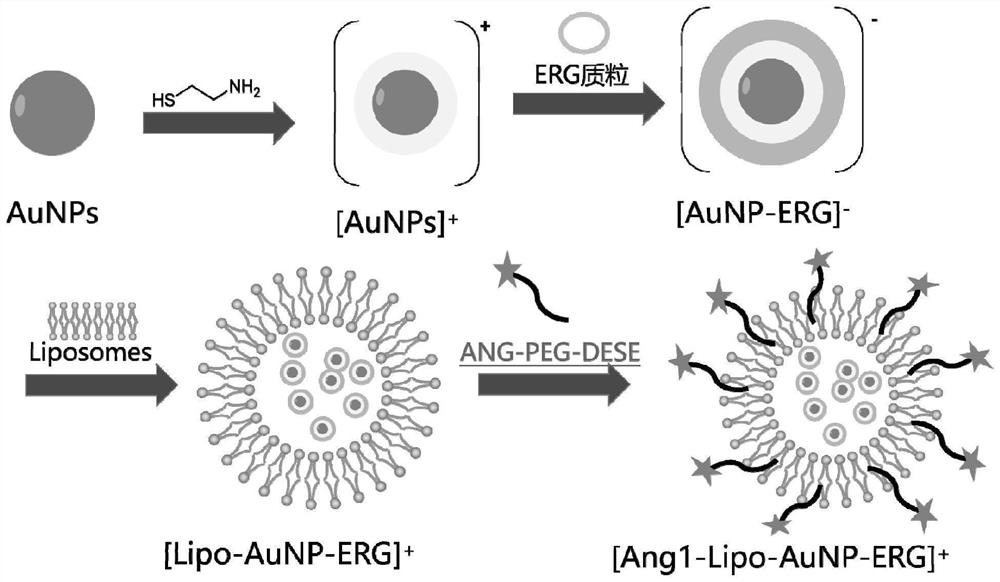
[0028] The invention provides a lipid-gold nanoparticle complex for treating cerebral edema by means of gene therapy, the complex is an Ang1-Lipo-AuNP-ERG complex, and the preparation method comprises:
[0029] Add sodium lauryl sulfate to the AuNPs solution for reaction; then add β-mercaptoethylamine for reaction; dialyze and purify the reacted solution, and the dialysate is a weakly acidic citric acid solution; filter after dialysis to obtain Cationic AuNPs solution;
[0030] Add cationic AuNPs solution to ERG plasmid solution and stir overnight to obtain AuNP-ERG solution;
[0031] DSPE-PEG 2000 -MAL and Ang-1 were reacted in solution, purified by dialysis to obtain Ang-DP 2000 ;
[0032] Take soybean lecithin, cholesterol and 2-dioleoyl hydroxypropyl-3-N,N,N-trimethylammonium chloride DOTAP respectively, add them to the methanol solution, heat to dissolve the three, mix well, and cool to room temperature , adding the Ang-DP 2000 Mix well, evaporate methanol to obtain ...
Embodiment 1
[0045] The lipid-gold nanoparticle complex for treating brain edema of the present invention includes the Ang1-Lipo-AuNP-ERG complex. The preparation steps of the complex are specifically:
[0046] (1) Preparation of AuNPs
[0047] The glass instruments used were soaked in aqua regia, washed with distilled water three times and dried for later use; 30 μL of gold tetrachloride solution with a mass fraction of 25.6% and 60 mL of distilled water were added to a 100 mL two-necked bottle, and heated to boiling. Then add 8 mL of 1% trisodium citrate solution, continue heating, the solution turns from light yellow to wine red, stop heating and cool to room temperature after boiling for 20 minutes, and obtain 13±2nm gold nanoparticles. Store at 4°C protected from light.
[0048] (2) Preparation of cationic AuNPs
[0049] Add 0.3 g sodium dodecyl sulfate SDS to the synthesized AuNPs solution and stir for 2 h. Subsequently, 0.5 g of β-mercaptoethylamine was added and stirring was co...
Embodiment 2
[0056] The lipid-gold nanoparticle complex for treating brain edema of the present invention includes the Ang1-Lipo-AuNP-ERG complex. The preparation steps of the complex are specifically:
[0057] (1) Preparation of AuNPs
[0058] All glass instruments used were soaked in aqua regia, washed three times with distilled water, and dried. Add 10 μL of gold tetrachloride solution with a mass fraction of 25.6% and 40 mL of distilled water into a 100 mL two-necked bottle, and heat to boiling. Then add 6 mL of 1% trisodium citrate solution, continue heating, the solution turns from light yellow to wine red, stop heating and cool to room temperature after boiling for 15 minutes, and obtain 13±2nm gold nanoparticles. Store at 4°C protected from light.
[0059] (2) Preparation of cationic AuNPs
[0060] Add 0.1 g sodium dodecyl sulfate SDS to the synthesized AuNPs solution and stir for 1 h. Subsequently, 0.3 g of β-mercaptoethylamine was added and stirring was continued for 2 h. P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com