Bifidobacterium longum subsp. Infantis CCFM1192 strain, leavening agent and preparation method and application thereof
A technology of CCFM1192 and CFM1192, applied in the field of microorganisms, can solve the problems of recurrent asthma, emphysema, and poor patient compliance, and achieve the effect of improving pathological characteristics, relieving asthma, and having great application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Screening, morphological observation and identification of Bifidobacterium longum subsp. infantis
[0055] Take 1g of fresh feces from healthy infants, spread it on mMRS solid medium containing 1% mupirocin after gradient dilution, and culture in an anaerobic environment at 37°C for 72 hours; Second, after culturing at 37°C for 48 hours, the colonies were observed and observed under a microscope, and it was found that the colonies were milky white, rounded, convex, smooth, and the shape of the bacteria was slightly irregular, with rounded ends, usually Single and small clusters exist.
[0056] The strains obtained from the above screening were cultivated in fresh mMRS liquid culture at 37°C for 24 hours, and then 1 mL of the bacterial liquid was centrifuged at 3500 × g to obtain the bacterial sludge, and then 1 mL of sterile water was added to wash the bacterial sludge, and the supernatant was removed by centrifugation under the same conditions. Then add 1...
Embodiment 2
[0064] Example 2: Culture of Bifidobacterium longum subsp. infantis
[0065] Introduce Bifidobacterium longum subsp. infantis CFM1192 into mMRS liquid medium and culture at 37°C for 24 hours, then transfer to fresh mMRS liquid medium, cultivate under the same conditions for 16-24 hours, centrifuge the bacteria at 6000×g for 15 minutes, and use 0.9 After washing the cells with % normal saline, they were centrifuged again at 6000×g for 10 min to collect the cells, resuspended with 30% (m / v) sucrose solution, and frozen in a -80°C refrigerator until use.
[0066] When the Bifidobacterium longum subsp. infantis strain is used for intragastric administration to mice, the supernatant is centrifuged after being taken out from a -80° C. refrigerator, and resuspended with normal saline to obtain the probiotic suspension used for intragastric administration.
Embodiment 3
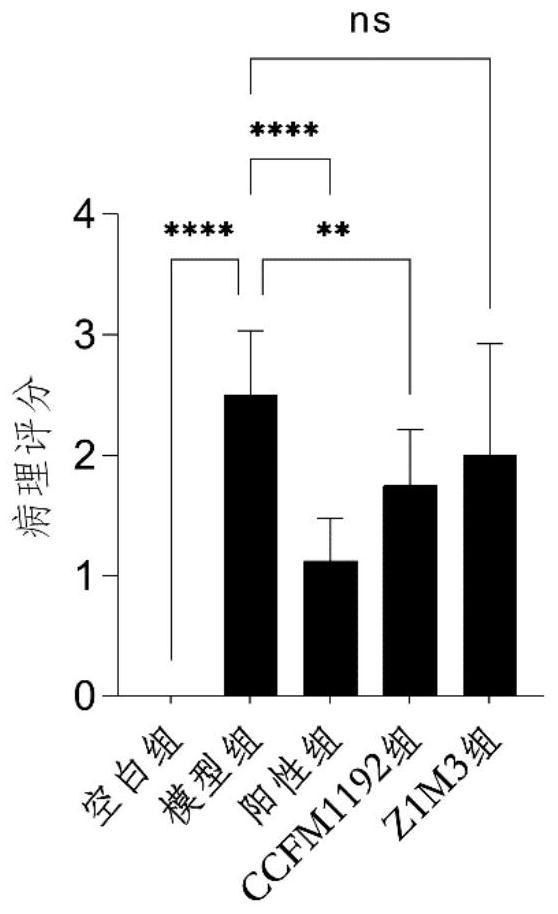
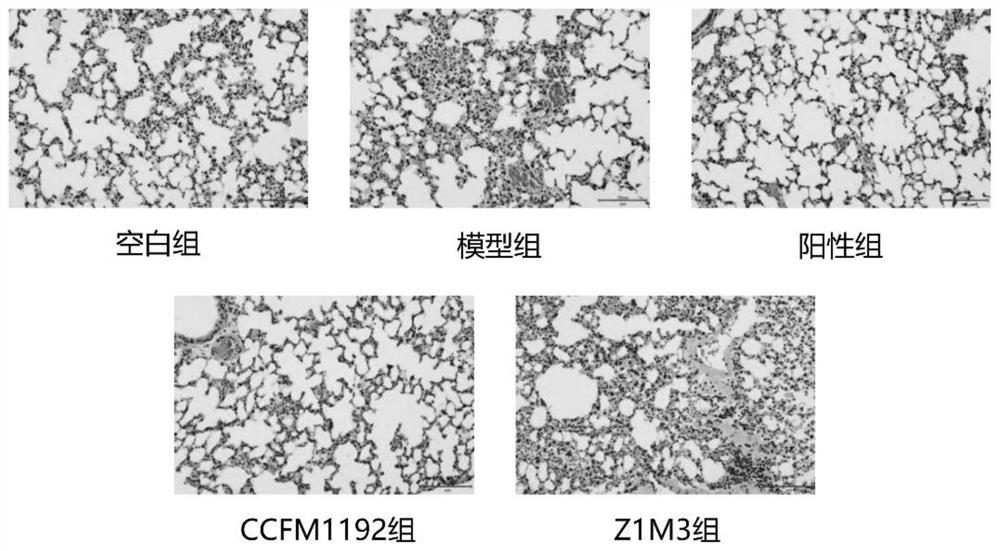
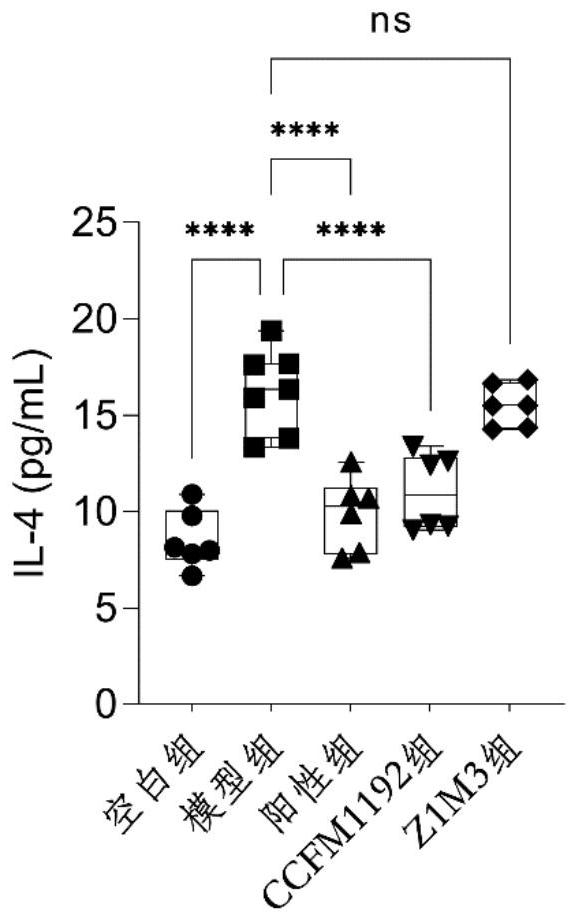
[0067] Example 3: Bifidobacterium longum subsp. infantis CCFM1192 significantly reduces pathology scores in asthmatic mice
[0068] The 6-week-old male SPF grade BABL / c mice were divided into 5 groups, namely blank group, model group, positive group (Lactobacillus reuteri CCFM1040), Z1M3 group and CCFM1192, with 8 mice in each group. Animal center, fed with common feed, constant temperature 21-26°C, humidity 40-70%, noise less than or equal to 60dB, animal illumination 15-20LX (all animal experiment procedures are reviewed and approved by the Animal Welfare and Ethics Management Committee of Jiangnan University) .
[0069] The experimental period was 5 weeks in total. The mice were administrated after 7 days of adaptation. From the 8th day to the 35th day, the blank group and the model group were administrated with 0.2 mL of normal saline solution, and the positive control group, CCFM1192 group and Z1M3 group were administrated with 0.2 mL of normal saline solution. The bac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com