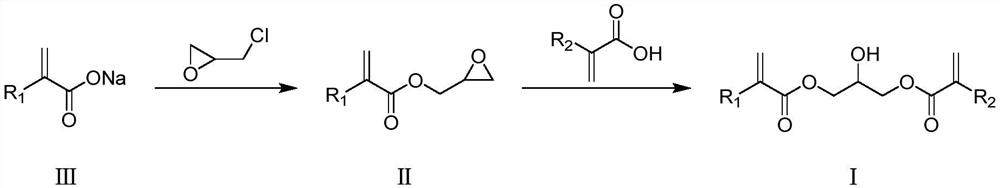
Preparation method of photoresist resin monomer containing hydroxyl structure
A technology of resin monomer and structured light, applied in the field of photoresist, can solve the problem of low preparation purity, and achieve the effects of high purity, high yield and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] first step
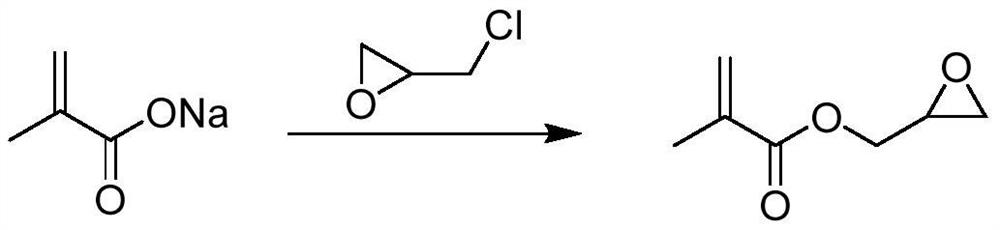
[0045] Reaction equation:
[0046]
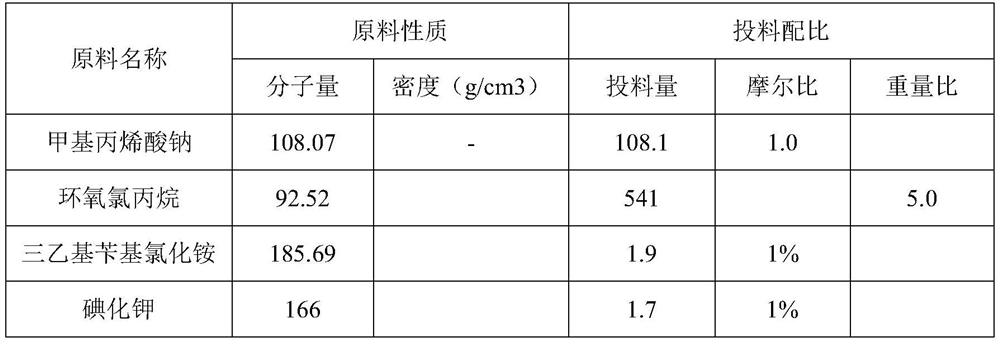
[0047] Material ratio
[0048]
[0049] Operation process:
[0050] Under nitrogen protection, sodium methacrylate (108.1g, 1mol), epichlorohydrin (541g, 5.85mol), triethylbenzyl ammonium chloride (1.9g, 0.01mol), potassium iodide (1.7g, 0.01mol) ), phenothiazine (2.0g) were added into a 1L four-necked reaction flask, the stirring was started, the temperature was raised to 110°C, and the reaction was continued at 110-120°C for 6 hours.
[0051] After the reaction was completed, the temperature was lowered to 20-30° C., and the solids were removed by filtration. After the filtrate decompressed to recover epichlorohydrin, the distillation was continued to obtain 86.7 g of colorless liquid glycidol methacrylate (purity GC=97.1%, yield Rate y = 61%)
[0052] In this example, sodium methacrylate is directly used for the reaction. Compared with methacrylic acid+alkali, the reactivity is better, the aftertreatment is si...
Embodiment 2
[0063] first step:
[0064] operation process
[0065] Under nitrogen protection, sodium methacrylate (108.1g, 1mol), epichlorohydrin (541g, 5.85mol), tetrabutylammonium bromide (3.36g, 0.01mol), potassium iodide (1.7g, 0.01mol), Phenothiazine (2.0 g) was added into a 1 L four-necked reaction flask, stirring was started, the temperature was raised to 120°C, and the reaction was continued at 110-120°C for 10 hours.
[0066] After the reaction was completed, the temperature was lowered to 20-30° C., the solids were removed by filtration, and the filtrate was decompressed to recover epichlorohydrin, and then continued to distill to obtain 85.8 g of colorless liquid glycidol methacrylate (purity GC=97.3%, yield rate y = 60.4%)
[0067] Step two:
[0068] Operation process:
[0069] Under nitrogen protection, glycidyl methacrylate (71.1g, 0.5mol), dioxane (300g), N,N-dimethylethylenediamine (0.44g, 0.005mol), antioxidant Add 264 (0.7g) into a 1L four-necked reaction flask, sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com