Respiratory pathogen multiplex detection kit and application thereof
A detection kit and respiratory technology, applied in the field of molecular biology, can solve problems such as complex operation process, manual on-duty, and long detection time, and achieve the effects of simplifying experimental operation steps, preventing environmental pollution, and improving detection throughput
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
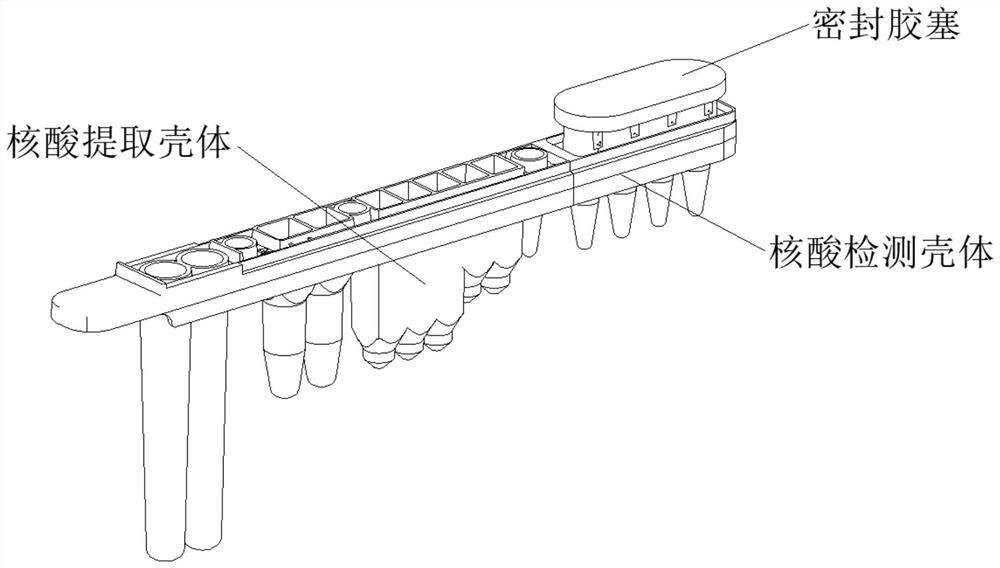
[0022] Respiratory pathogen multiple detection kit, said kit includes specific primers and fluorescent probes for amplifying five gene loci of influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus and mycoplasma pneumoniae; Among them, the 5 loci are: M gene of influenza A virus, NS gene of influenza B virus, M gene of respiratory syncytial virus, H gene of adenovirus, Mgp gene of Mycoplasma pneumoniae; and human reference gene RP specific primers and fluorescent probes.
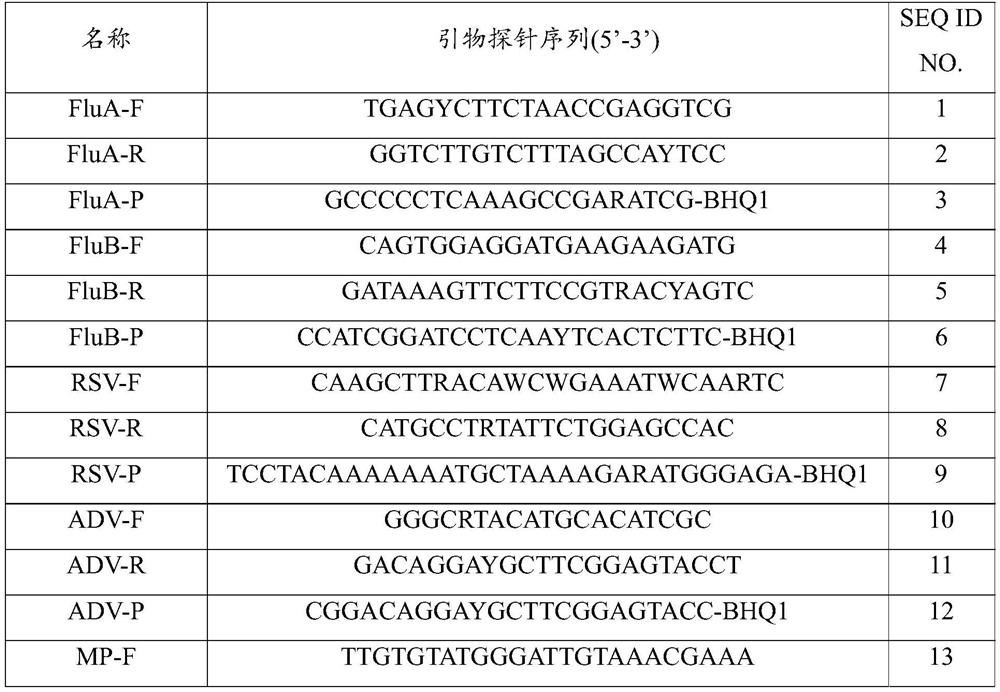
[0023] Preferably, the sequences of the specific primers and fluorescent probes are as follows: FluA, SEQ ID NO.1-3; FluB, SEQ ID NO.4-6; RSV, SEQ ID NO.7-9; ADV, SEQ ID NO. 10-12; MP, SEQ ID NO. 13-15; Homo, SEQ ID NO. 16-18.
[0024] Table 1 Specific primers and fluorescent probe sequence list
[0025]
[0026]
[0027] Preferably, the kit includes PCR reaction solution I and PCR reaction solution II; wherein, PCR reaction solution I includes SEQ ID NO.1-2, SEQ ID NO.4-5, SE...
Embodiment 2
[0032] The influenza virus ribonucleic acid (IVARNA) liquid indoor quality control product, the influenza A H5N1 influenza virus ribonucleic acid (H5N1RNA) liquid indoor quality control product, the influenza A H7N9 influenza virus ribonucleic acid (H7N9RNA) liquid indoor quality control product, H9N2 influenza virus ribonucleic acid (H9N2RNA) liquid indoor quality control material, influenza B virus ribonucleic acid (IVBRNA) liquid indoor quality control material, respiratory syncytial virus type B ribonucleic acid liquid indoor quality control material, human metapneumovirus RNA ( HMPVRNA) liquid indoor quality control, adenovirus deoxyribonucleic acid (ADVDNA) liquid indoor quality control, adenovirus type 11 deoxyribonucleic acid (ADV11DNA) liquid indoor quality control, mycoplasma pneumoniae deoxyribonucleic acid (MPDNA) liquid indoor quality control Add human parainfluenza type 1 virus ribonucleic acid (PIV1RNA) liquid indoor quality control product, human parainfluenza t...
Embodiment 3
[0038] Embodiment 3 Sensitivity detection test
[0039] Dilute the plasmid DNA containing the respective targets of influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus and mycoplasma pneumoniae with a certain number of copies in sterilized purified water, and then add them to the detection wells of the detection device on the detection.
[0040] It can be shown from the detection results that the detection sensitivity of the detection method for influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus and Mycoplasma pneumoniae is 500 copies / reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com