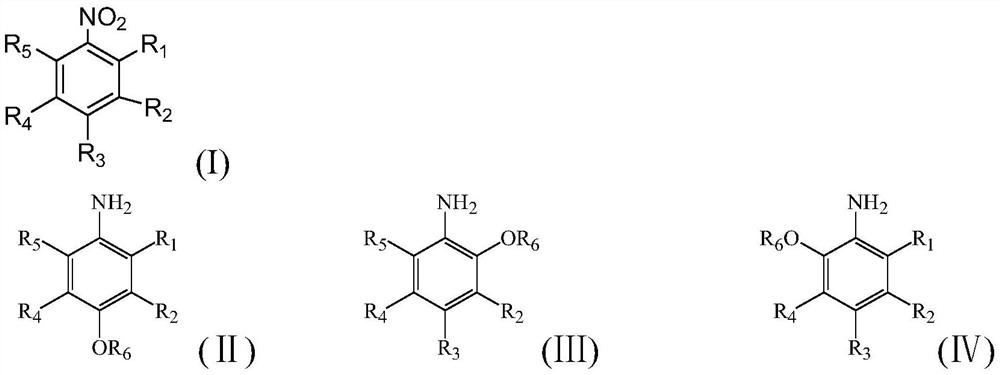
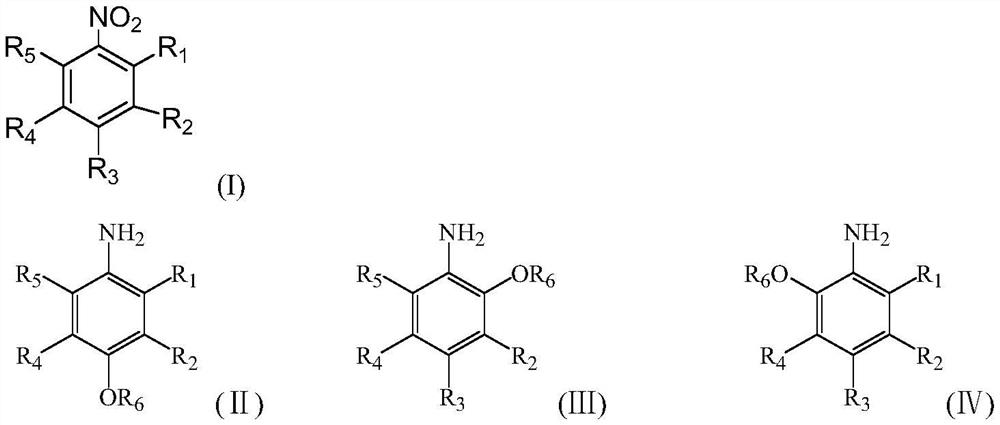
Supported carbon-coated bimetallic catalyst and application thereof
A bimetallic catalyst, supported technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of easy loss of precious metals, loss of precious metals, irreversible deactivation of catalysts, etc. problems, to achieve the effect of improving catalytic activity and target product selectivity, good stability, and high target product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Weigh 10g of commercially available 3%Pt-1%Ru / C, add it into 15ml of water and mix evenly, add 20g of 20wt% lactose aqueous solution and 5g of 10wt% hydroxyethyl cellulose aqueous solution in turn, soak at room temperature for 2h , vacuum-dried at 40°C for 20h to remove moisture, and then it was calcined at 400°C under nitrogen for 20h at high temperature; then, the temperature was lowered to 100°C and treated in air atmosphere for 10h to obtain a supported carbon-coated platinum- Ruthenium catalyst.
Embodiment 2
[0049] Weigh 10g commercially available 5%Pt-0.5%Au / TiO 2 , add it into 15ml of water and mix evenly, add 20g concentration of 20wt% glucose aqueous solution and 5g concentration of 20wt% hydroxyethyl cellulose aqueous solution, after soaking at room temperature for 2h, vacuum dry at 60°C for 10h, remove moisture, and then put it in High-temperature calcination at 900° C. for 2 h under hydrogen; then, the temperature was lowered to 180° C., and treated in an air atmosphere for another 4 h to obtain a supported carbon-encapsulated platinum-gold catalyst.
Embodiment 3
[0051] Weigh 10g of commercially available 0.5%Pt-1%Ag / diatomaceous earth, add it into 15ml of water and mix evenly, add 5g of 20wt% sucrose aqueous solution and 5g of 15wt% hydroxyethyl cellulose aqueous solution, soak at room temperature for 2h Afterwards, it was vacuum-dried at 20°C for 40 hours to remove moisture, and then it was roasted at 600°C for 4 hours in a vacuum state; then, the temperature was lowered to 150°C and treated in an air atmosphere for 6 hours to obtain a supported carbon package. Platinum-silver catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com