Solid phosphazene compound, preparation method and application
A technology of compound and phosphazene, which is applied in the field of preparation and solid phosphazene compounds, can solve the problems of reduced catalytic activity, fewer types, complicated post-processing steps, etc., achieve low raw material cost, simple preparation process, and reduce environmental impact Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In a second aspect, the present invention provides a method for preparing the above-mentioned solid phosphazene compound, comprising:
[0037] 1, phosphorus pentachloride contacts with the compound shown in formula (2), then contacts with ammonia gas, finally contacts with alkali solution, so that obtain the compound shown in formula (3);
[0038]
[0039]
[0040] HN=PR 3
[0041] Formula (3);
[0042] Ⅱ. Hexachlorocyclotriphosphazene is contacted with a crosslinking agent and an acid-binding agent to obtain a compound shown in formula (4);
[0043]
[0044] III. The compound represented by formula (1) can be prepared by contacting and reacting the compound of formula (3), the compound of formula (4) and the acid-binding agent.
[0045] In a typical embodiment, in step I, the contacting is carried out in an anhydrous organic solvent, further, the anhydrous organic solvent is selected from at least one of dichloromethane, acetonitrile, toluene, tetrahydrofur...
Embodiment 1
[0077] Preparation of PZS microspheres
[0078]
[0079] Dissolve 1.78g of hexachlorocyclotriphosphazene and 2.55g of triethylamine in 255ml of acetonitrile as solution A, and dissolve 2.55g of 4,4'-dihydroxydiphenylsulfone in 255ml of acetonitrile as solution B. Add the B solution to the A solution dropwise under the conditions, control the system temperature at 35545°C, react for 3 hours after the dropwise addition is completed, filter and separate the solid phase after the reaction, wash it with absolute ethanol and deionized water several times, and place it in a vacuum After drying in an oven at 65°C, 3.47 g of the product was recovered, with a yield of 85.32%.
Embodiment 2
[0081] Preparation of Tris(tetramethylguanidine)phosphazene
[0082]
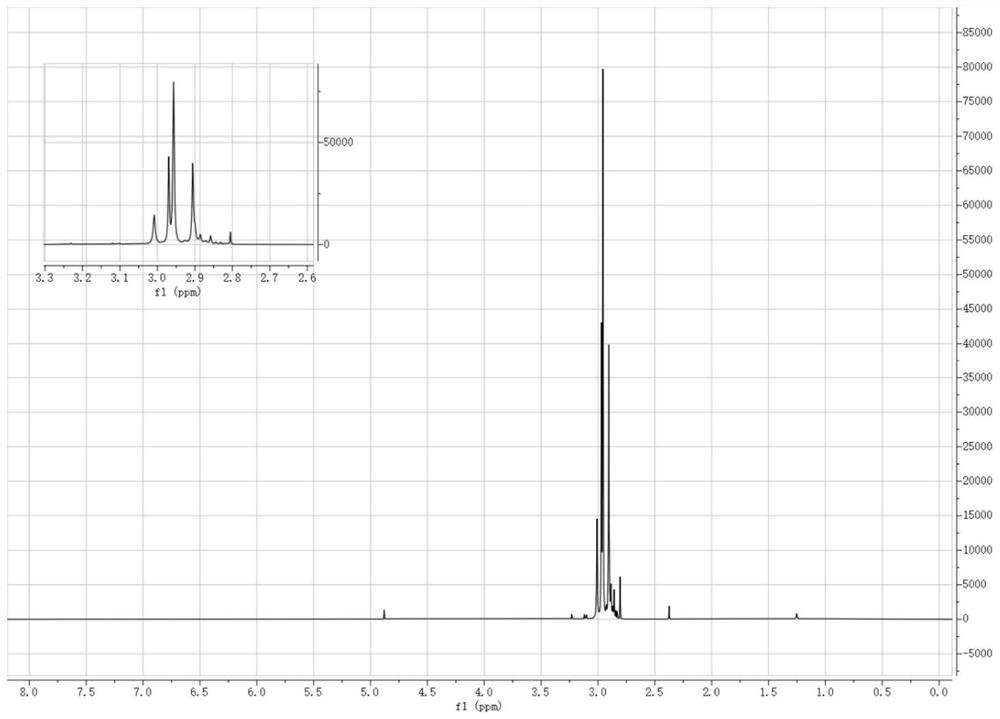
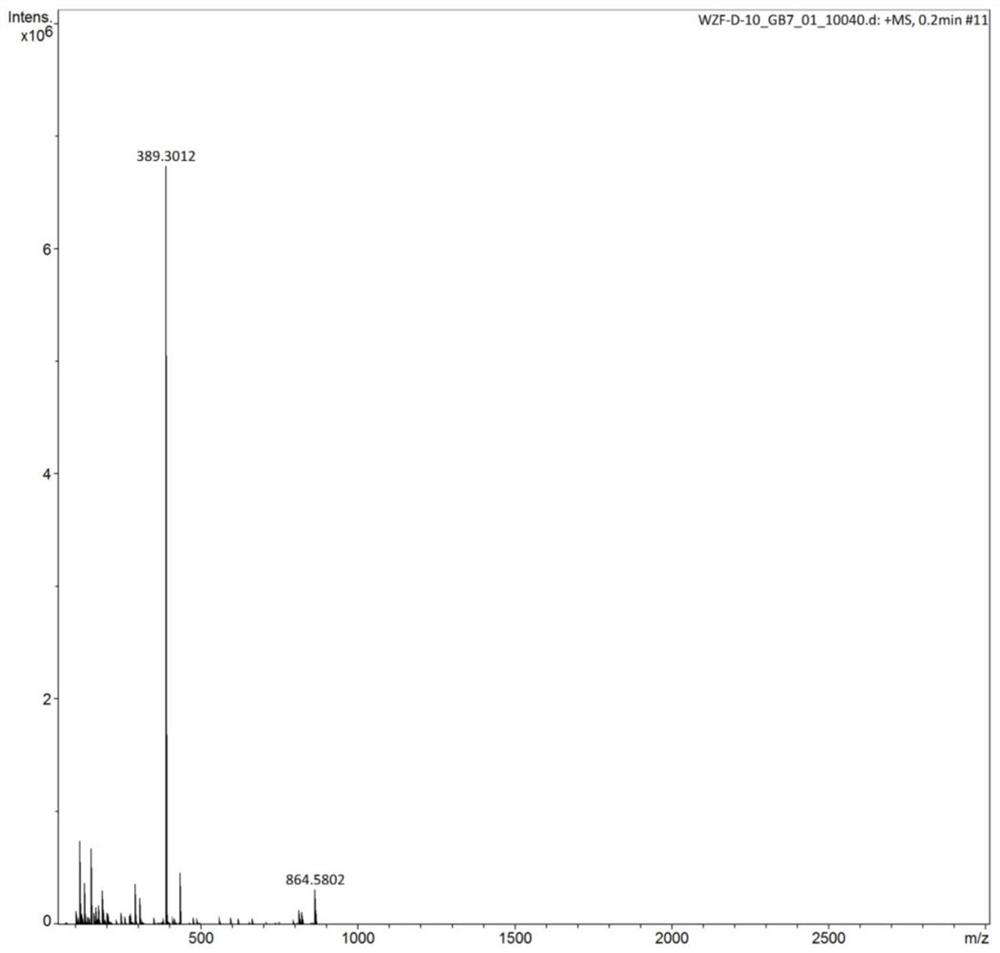
[0083] Dissolve 25.824g of phosphorus pentachloride in 55ml of anhydrous dichloromethane in a three-necked flask, place it in a cold bath at -25°C under the protection of argon, and add 51.75g of tetramethylguanidine dropwise into the system through a constant pressure funnel , Reaction 1h after dropwise addition. After continuing to react at room temperature for 2 h, the temperature of the system was lowered to -25°C again, and ammonia gas was continuously fed into the system until it was saturated. After reacting for 1 h, continue to react with ammonia gas for 3 h at room temperature until no obvious precipitation is formed on the surface after the stirring is stopped. Remove the solid phase by filtration, remove the solvent by rotary evaporation, then add the obtained solid to 45ml of 55wt% NaOH aqueous solution, mix and stir for 12h under the protection of argon, remove the solid phase by filtrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com