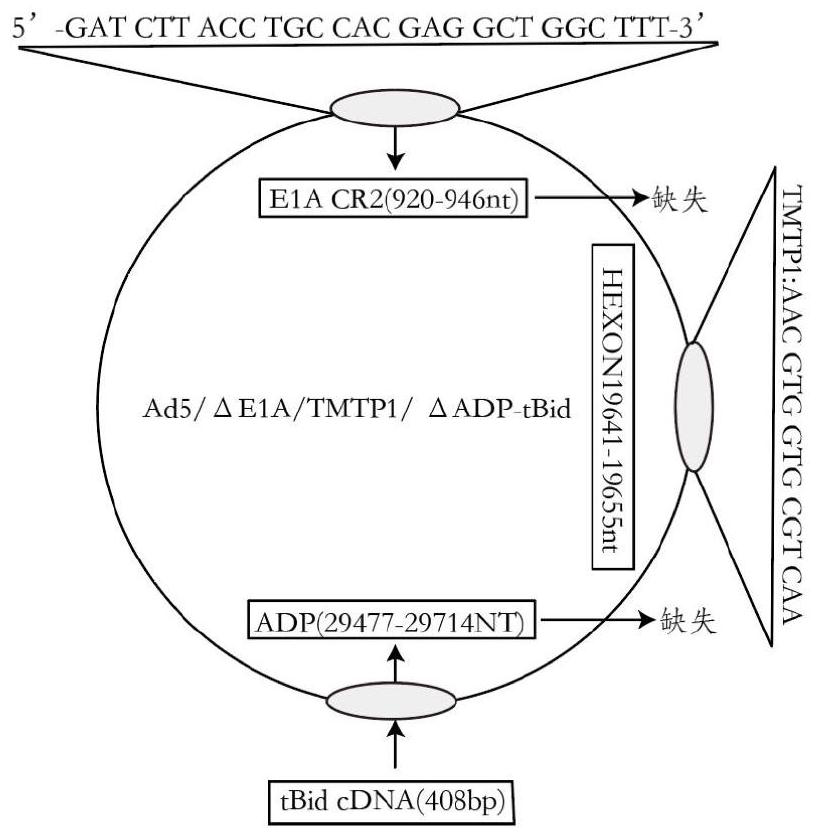
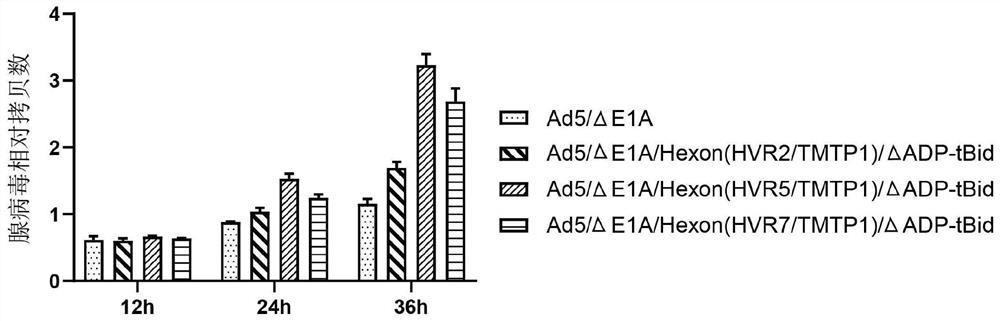
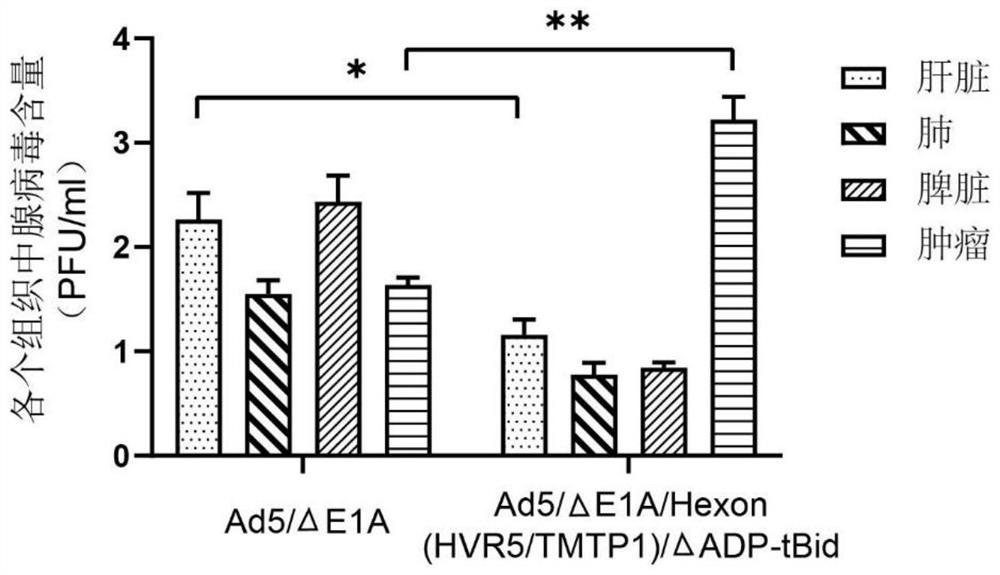
Oncolytic adenovirus recombinant carrying TMTP1 and tBid as well as construction method and application of oncolytic adenovirus recombinant
An oncolytic adenovirus and recombinant technology, applied in the field of medical genetic engineering, can solve problems such as the unfavorable killing effect of adenovirus, and achieve the effect of positive pharmaceutical value and broad social significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Construction of pAd5 / ΔE1A adenovirus packaging plasmid
[0060] Step 1.1: Construction of Shuttle Plasmid Blunt-Zero-E1A / ΔE1A
[0061] The pXC1-ΔE1A plasmid is a deletion of 27 bases in the 920nt-946nt region of the E1A conserved sequence 2 region of the human type 5 adenovirus gene, as shown in SEQ ID NO.1. Its construction method is as follows:
[0062] The pXC1 plasmid was purchased from Microbix Biosystem Inc. (Toronato, Ontario, Canada, Cat. No.: PD-01-03), which contains the human adenovirus type 5 (Ad5) 22nt-5790nt sequence. The 920nt-946nt region was deleted by 3 times PCR.
[0063] Acquisition of Fragment 1: Primer 1: 5′-cg ggatcc gggcccccatttcc-3' (SEQ ID NO 2), corresponding to 9883-9902nt, the underlined part is the BamHI restriction site; primer 2: 5'- gtcactgggtggatcgatcacctc cggtac-3' (SEQ ID NO 3), corresponding to 922nt-905nt, the underlined part is complementary to primer 3; PCR reaction was carried out with pXC1 as template, the total...
Embodiment 2
[0076] Example 2: Construction of pAd5 / ΔE1A / Hexon (HVR / TMTP1) plasmid vector
[0077] Step 2.1: Construction of the shuttle vector pEASY-Blunt-Zero-Hexon(HVR / TMTP1)
[0078] pBHGE3 was purchased from Microbix Biosystem Inc. (Toronato, Ontario, Canada, catalog number: PD-01-12), this plasmid contains the entire genome sequence except the Ad5 packaging signal (194-358nt). When pBHGE3 was obtained from MicrobixBiosystem Inc., the total amount was 10 μg. First, it was electroporated into competent bacteria, positive clones were picked, and plasmids were extracted. The obtained plasmids were purified by CsCl2-EB ultracentrifugation. Homologous recombination method to obtain Δ920-946Ad5 recombinant adenovirus construct, the method is as follows:
[0079] Plant 7.5×10 in a 15cm petri dish 5 293 cells, the culture medium is 10% FBS DMEM, by the next day, the cells should be 1-1.5×10 6 , about 70% of the cells were confluent; 3-4 hours before transfection, replace with fresh culture...
Embodiment 3
[0083] Example 3: Construction of Ad5 / ΔE1A / TMTP1 / ΔADP-tBid adenoviral vector
[0084] Step 3.1 Construction of adenovirus E3 region shuttle vector
[0085] Using the adenovirus Δ920-946Ad5 as a template (as described above, including the complete sequence of the adenovirus E3 region), a PCR reaction was carried out, the upstream primer: 5'-tgtcaccactaactgctttactcg-3' (SEQ ID NO 16), the downstream primer: 5'- gctgccctgcgtctttcta-3' (SEQ ID NO 17), the 26342-31140 fragment (ie the complete fragment of the E3 region) was obtained, which was ligated into the pEASY-Blunt-Zero vector to obtain the pEASY-Blunt-Zero-E3 plasmid.
[0086] Step 3.2: Plasmid pcDNA3.1-E3 / ΔADP is a backbone plasmid, in which the complete adenovirus E3 region is inserted but 29477bp-29714bp (the fragment between the two EcoRI restriction sites of adenovirus, ie the ADP region) is deleted, and Fragments with EcoRI restriction sites at both ends. Its construction method is as follows:
[0087] The Ad5 E3 r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com