Tripterine derivative and application thereof in tumor resistance
A technology for triptolide and derivatives, which is applied in the field of biomedicine, can solve the problems of low water solubility, high myocardial toxicity, influence on application, etc., and achieves broad application prospects and the effect of reducing cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
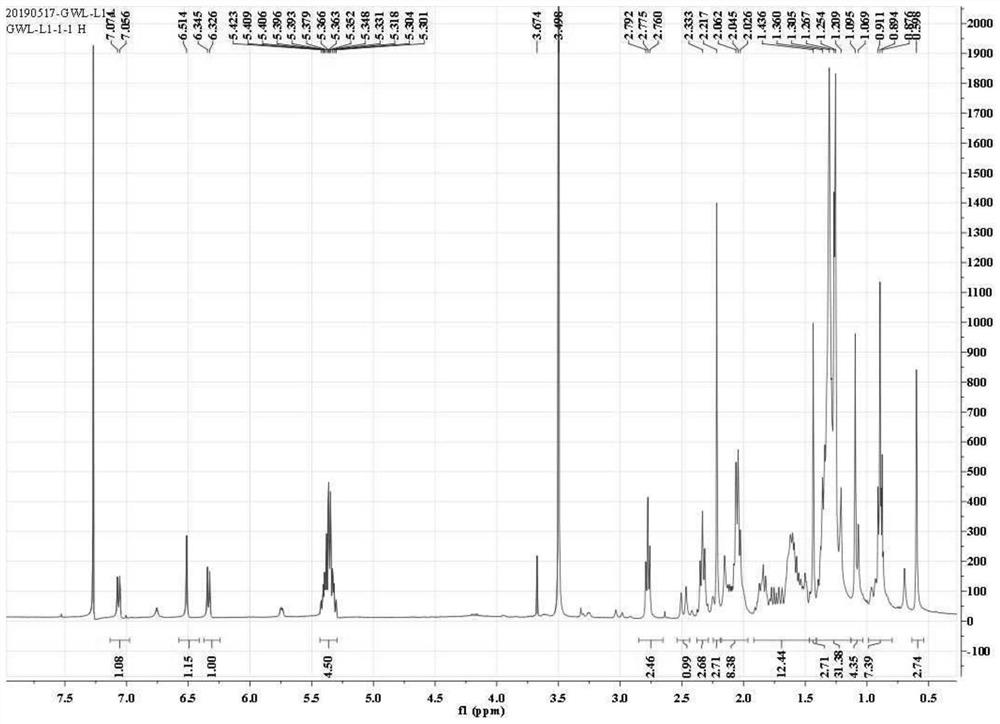
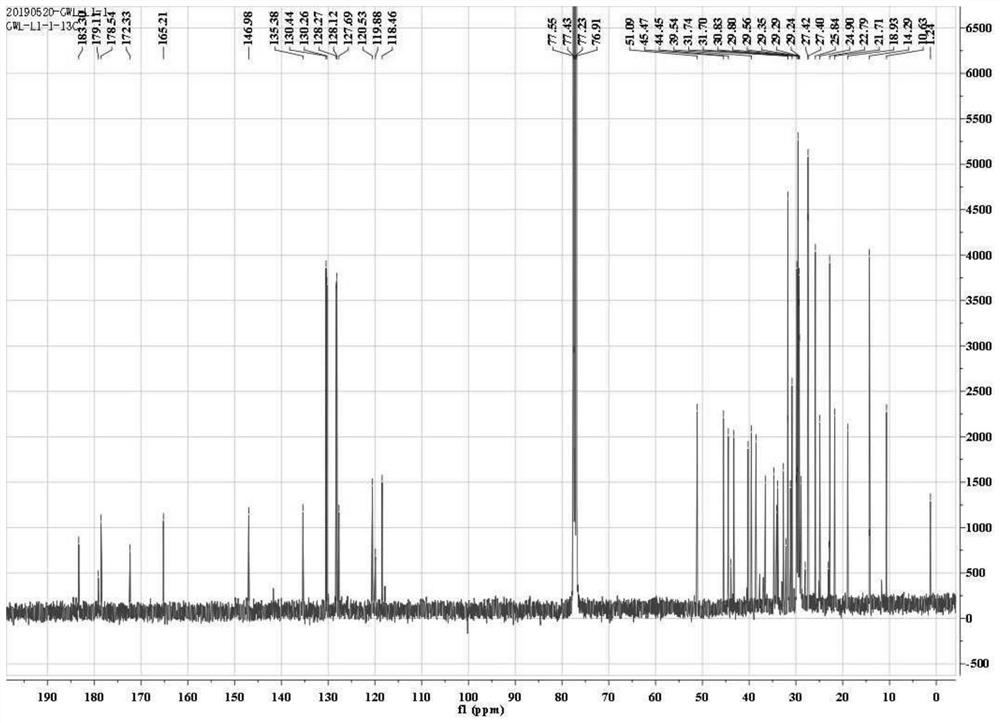
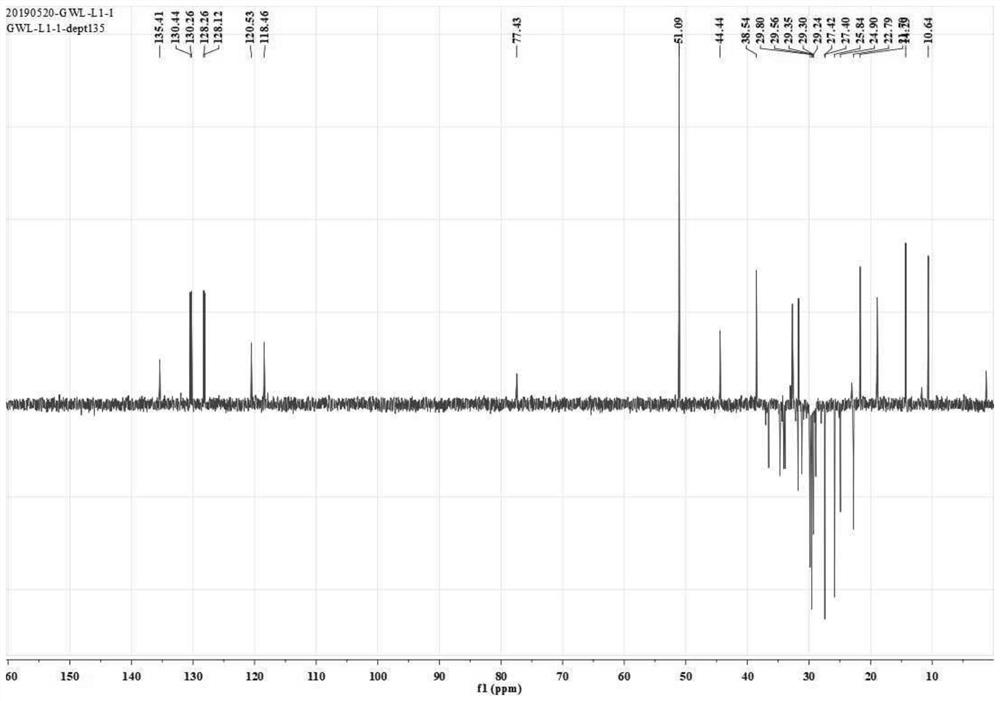
[0036] This example shows the application of Phomopsis LGT-5 to transform tripterine, and the tripterine derivative (compound 1) shown in formula (I) was obtained after separation and identification.
[0037] 1) Biological preparation
[0038] Phomopsis LGT-5 was inoculated into a Erlenmeyer flask containing 150 mL of modified Martin liquid medium, and cultured with shaking at 28°C and 180 rpm until it reached the logarithmic growth phase (usually the culture time was 1 day), at which point Phomopsis LGT The concentration of -5 is about 5g dry weight / L culture system. The dry weight measurement method used in this example is as follows: sampling the culture system, collecting the bacteria by centrifugation, washing thoroughly with sterile water, and then drying to dry weight. The formula of the improved Martin's liquid medium (pH 6.2-6.6) described in this example is: 5 g of peptone, 1 g of dipotassium hydrogen phosphate, 0.5 g of magnesium sulfate, 2 g of yeast extract powde...
Embodiment 2
[0055] In this example, a cytotoxicity test was carried out to measure the antitumor activity of Compound 1, and the results of the determination are shown in Figure 9-Figure 13 .
[0056] Test agent: Celastrol (tripterine), compound 1 prepared in Example 1.
[0057] Test cells: U251 cells, A549 cells, KG-1 cells, B16 cells; BV-2 cells; H9c2 cells; PC12 cells.
[0058] Preparation of the test agent solution: Dissolve the test agent in DMSO to obtain a solution with a concentration of 50mmol / mL, then dilute it with complete medium to obtain the test agent with a concentration of 8μmol / L, 5μmol / L, 2μmol / L or 1μmol / L. Test the test drug solution. The complete medium consists of 9 volume parts of DMEM medium and 1 volume part of fetal calf serum.
[0059] Culture conditions: 37°C, 5% CO2.
[0060] experiment procedure:
[0061] 1) Take a 96-well plate and add 100 μL of the cell suspension (containing 5×10 3 cells to be tested), cultivated for 24 hours, and discarded the sup...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com