Engineered exosome in-situ nano vaccine as well as preparation method and application thereof
A nano-vaccine and exosome technology, applied in biochemical equipment and methods, chemical instruments and methods, and botanical equipment and methods, can solve the problems of poor efficacy of adjuvant radiotherapy and chemotherapy, and achieve the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0038] Example 1: Preparation of α-LA engineered exosome in situ nanovaccine encapsulating ELANE and Hiltonol
[0039] A method for preparing an α-LA engineered exosome in situ nano-vaccine wrapping ELANE and Hiltonol, the method comprising the following steps:
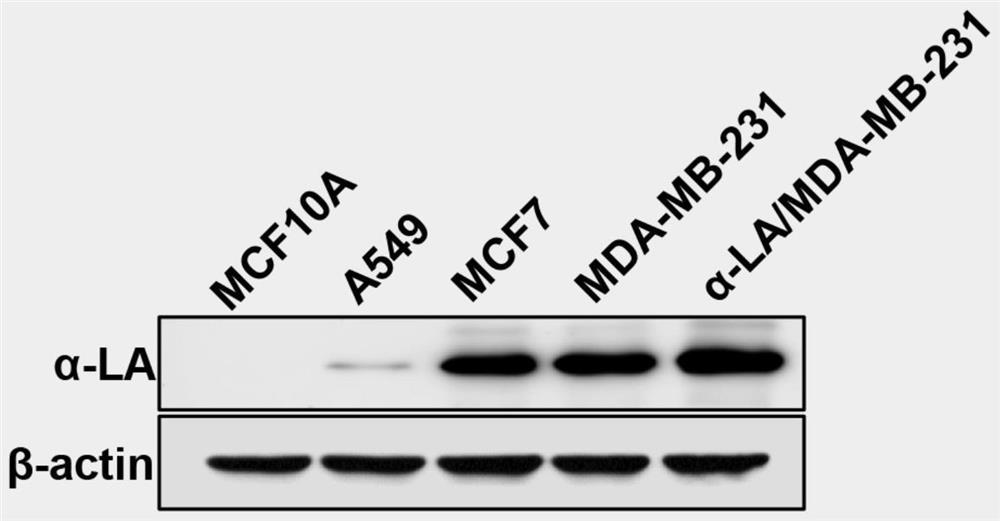
[0040] 1. Construction of MDA-MB-231 cell line stably overexpressing α-LA
[0041]The full-length coding sequence of human α-LA (Accession: CR542017) was cloned from the total RNA of MDA-MB-231 cells (preserved in our laboratory), and cloned into the lentiviral expression vector pCDH-CMV-puro (JHepatol.2017Oct ; 67(4):739-748.) (preserved by our laboratory). Human 293T cells were inoculated in a 6cm dish and cultured for 24h, and then were mixed with pCDH-CMV-puro-α-LA, pMD2.G (J GeneMed.2018Jul; 20(7- 8):e3027.) (preserved in our laboratory) and psPAX2 (JGene Med.2018Jul; 20(7-8):e3027.) (preserved in our laboratory) plasmids were co-transfected into human 293T cells. Virus was harvested after 48 hours and titrate...
Embodiment 2
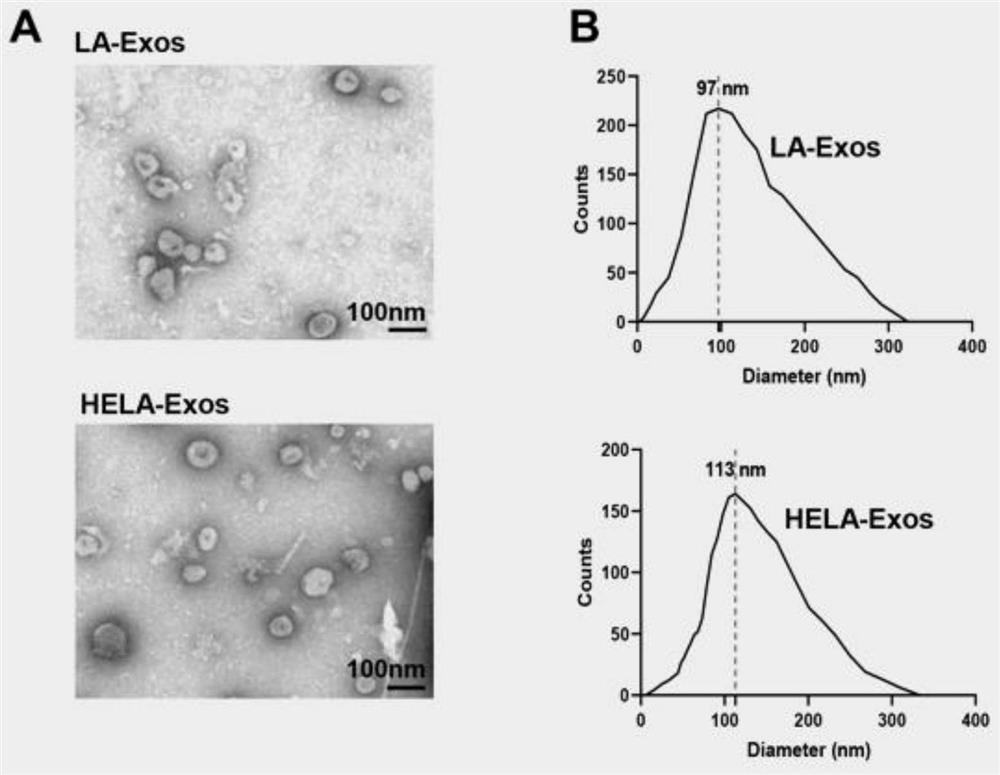
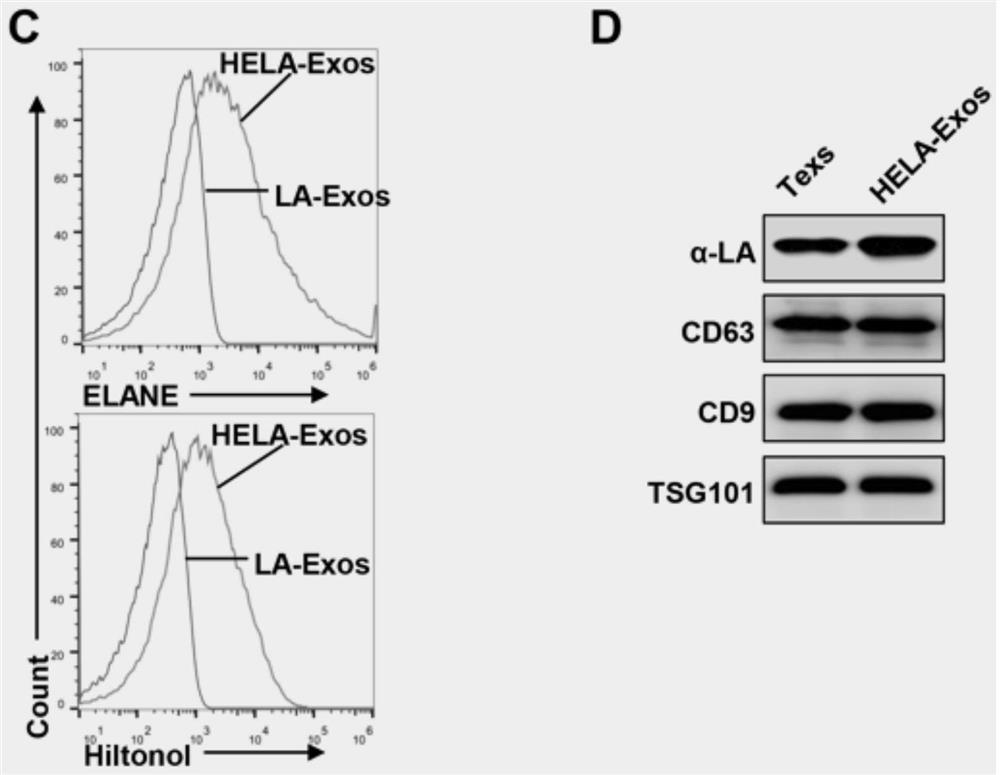
[0046] Example 2: In vitro biological function evaluation of nano-vaccine HELA-Exos
[0047] The in vitro biological function evaluation of nanovaccine HELA-Exos includes the following three parts:
[0048] 1. In vitro cell targeting ability evaluation of nano-vaccine HELA-Exos
[0049] HELA-Exos or Texs were labeled with DiI and incubated with a cell mixture of MDA-MB-231 cells and human peripheral blood mononuclear cells (PBMC) for 2 hours, or after MDA-MB-231 cells and human lung cancer cells A549 cells were plated, respectively Add DiI-labeled HELA-Exos or Texs, and evaluate the cell uptake efficiency by flow cytometry and fluorescent confocal experiments. The results are as follows: image 3 As shown in A-B, HELA-Exos significantly improved the targeting ability compared with Texs, and MDA-MB-231 cells took up more nano-vaccine HELA-Exos compared with PBMC and A549 cells, indicating that HELA-Exos has the effect on breast cancer cells Has excellent target specificity. ...
Embodiment 3
[0054] Example 3: In vivo anti-cancer effect and immune activation evaluation of nano-vaccine HELA-Exos
[0055] The in vivo anti-cancer effect and immune activation evaluation of nano-vaccine HELA-Exos includes the following three parts:
[0056] 1. Evaluation of tumor growth inhibition of nano-vaccine HELA-Exos in immunocompetent orthotopic mice
[0057] Immunocompetent Balb / c mice were orthotopically inoculated with the MDA-MB-231 cell line stably expressing luciferase, and the tumors grew to about 100 mm in size after about 21 days 3 DMEM basal medium, Hiltonol or HELA-Exos treatment was started at the beginning of the treatment, and the tumor volume was monitored. After 30 days of treatment, the mice were anesthetized for in vivo imaging, and then the mice were sacrificed. The result is as Figure 5 Shown, in vivo tumor imaging and tumor growth curve results Figure 5 A-C show that HELA-Exos treatment significantly inhibits tumor growth in mice. In addition, in HELA-E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com