Albumin binding type camptothecin derivative prodrug as well as preparation method and application thereof
A technology of albumin-binding type and camptothecin, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of low drug loading dose, inability to directly administer drugs, low water solubility, etc., and achieve extended In vivo half-life, excellent anti-tumor activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of Compound Ia
[0045]
[0046] Preparation of compound IIa:
[0047] Under nitrogen protection conditions, 2.47g (5.10mmol) of compound 1, 2g (5.10mmol) of SN38 and 3.85g (15.30mmol) of ADDP were dissolved in 500mL of toluene, and 3mL of n-Bu 3 P (15.30mmol), heated at 80 degrees overnight. Spin dry toluene, dissolve in 500mL ethyl acetate, saturated NaHCO 3 solution (250 mL), washed with saturated brine (200 mL), dried over anhydrous sodium sulfate, and spin-dried. Add 50 mL of ethyl acetate to make a slurry, filter, and collect the filter cake as compound IIa, 3 g of light yellow solid, with a yield of 69%.
[0048] 1 H-NMR (400MHz, CDCl 3 )δ(ppm): δ8.17(d, J=9.2Hz, 1H), 7.97(d, J=2.1Hz, 1H), 7.69(dd, J=8.7, 2.2Hz, 1H), 7.62(s, 1H), 7.49(dd, J=9.3, 2.7Hz, 1H), 7.44(d, J=8.6Hz, 1H), 7.34(d, J=2.7Hz, 1H), 5.72(d, J=16.3Hz, 1H),5.44–5.14(m,9H),4.24(d,J=8.7Hz,1H),4.00(s,1H),3.74(s,3H),3.12(q,J=7.6Hz,2H), 2.13(s,3H),2.07(s,3H),2.06(s,3H),1.88(ddt,J=...
Embodiment 2
[0066] Synthesis of Compound Ib
[0067]
[0068] The preparation of compound Ib:
[0069] Under nitrogen protection, 0.80 g (1.00 mmol) of compound VIa, 0.31 g (1.20 mmol) of compound VIIb and 0.50 g (0.25 mmol) of sodium ascorbate were dissolved in 50 mL of tetrahydrofuran. 0.63g (0.25mmol) of copper sulfate pentahydrate was dissolved in 50mL of water and added to the reaction system. Stir at room temperature for half an hour. Prepare the liquid phase directly over half. The liquid phase method is the same as above. The effluent with a relative retention time of 9-10 min was collected and freeze-dried to obtain 0.96 g of compound Ib as a light yellow solid, with a yield of 90%. 1 H NMR (400MHz,DMSO)δ9.17(s,1H),8.36–8.20(m,2H),8.06(d,J=9.1Hz,1H),7.93(s,1H),7.59–7.48(m, 2H),7.33–7.21(m,2H),7.13(d,J=8.3Hz,1H),7.01–6.91(m,3H),5.41(s,2H),5.26(d,J=6.7Hz,4H ),4.87(d,J=7.4Hz,1H),4.40(t,J=6.6Hz,2H),4.27(s,2H),3.37–3.29(m,4H),3.17–3.10(m,2H) ,2.46–2.38(m,2H),2.17–2.00(m,5H),...
Embodiment 3
[0072] Compound Ia and plasma protein binding experiment
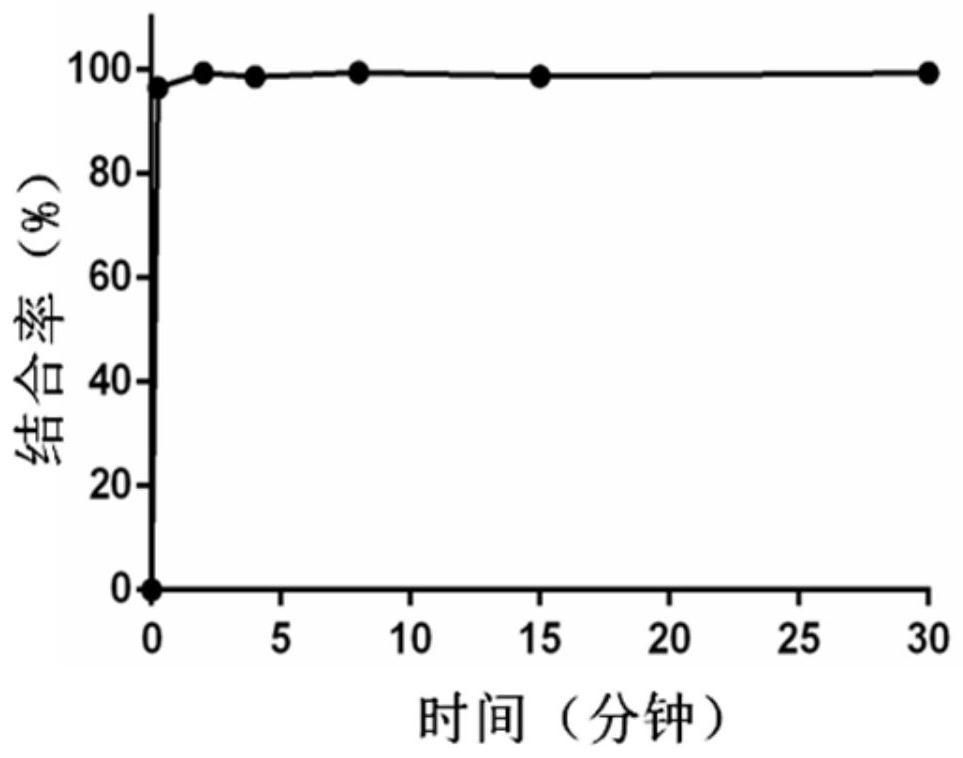
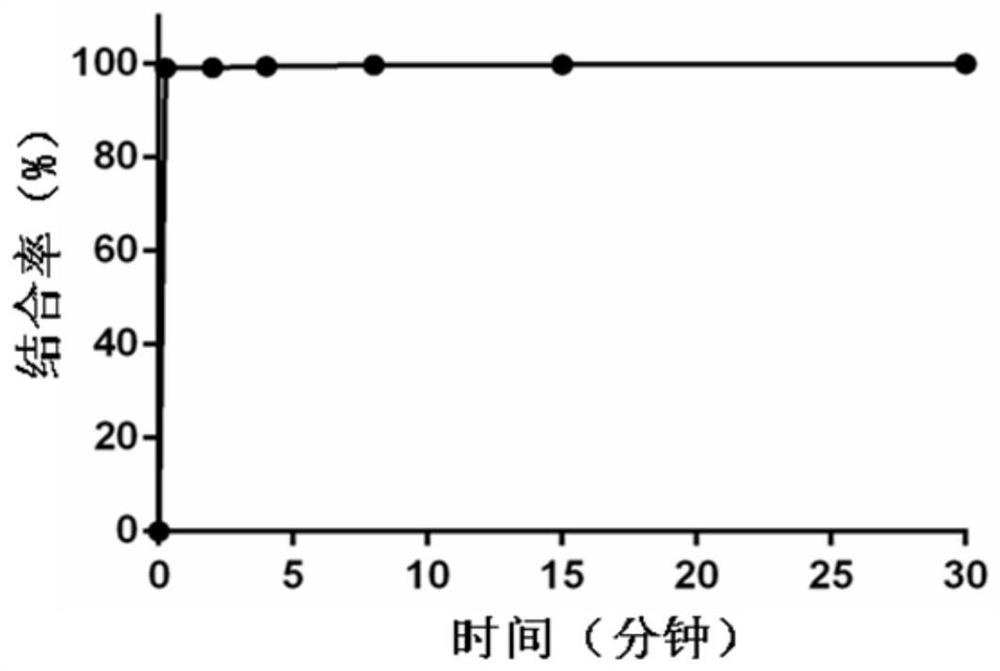
[0073] The plasma was warmed at 37°C for half an hour, and 6 μl of 10 mM compound Ia stock solution was added to 594 μl of plasma. Sampling time points are: 15s, 2min, 4min, 8min, 15min, 30min. Take 30 μl of the above plasma each time, add 120 μl of iced methanol-acetonitrile (v / v, 1:1) solution, vortex for 1 minute, and centrifuge at 9000 rpm at 4 degrees for half an hour. The supernatant was fed into the liquid phase to determine the compound Ia not bound to the protein. The experimental results are shown in the attached picture figure 1 and figure 2 . Within 2 minutes, 99% of compound Ia combined with plasma albumin, indicating that compound Ia can quickly combine with plasma albumin to form a macromolecule drug-carrying system after intravenous injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com