Pegylated F polypeptide as well as preparation method and application thereof
A PEGylation and polypeptide technology, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of increasing the biological inhibitory activity of polypeptides, and achieve the effect of strong inhibitory activity of HeLa cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis method of pegylated F polypeptide
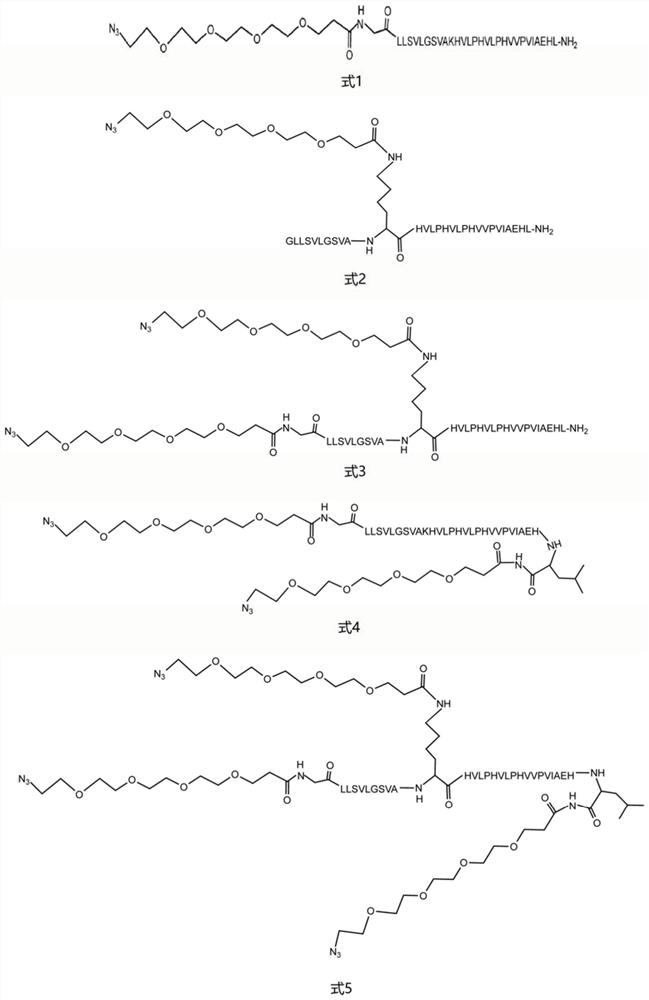
[0054] Because there are multiple amide groups on the F polypeptide sequence, NHS-PEG with amide group reactivity was selected. 4 -Azide molecules carry out the PEGylation reaction of F polypeptide, the specific steps are as follows:
[0055] 1) A certain amount (1mg) of NHS-PEG 4 - Azide was dissolved in dimethyl sulfoxide (20 μL) to prepare a 250 mM stock solution;
[0056] 2) Dissolving 5 mg of F1 or F3 polypeptide in PBS to prepare a 5 mg / mL solution;
[0057] 3) Fully mix the polypeptide solution prepared in 2) with the PEG solution in 1) at a molar ratio of 1:20, and then react the mixture at room temperature for 1 hour;
[0058] 4) Then add 1M Tris-HCl (trishydroxymethylaminomethane hydrochloride) buffer solution to quench until the final concentration of Tris-HCl is 50-100mM, and incubate the reaction at room temperature for 5 minutes;
[0059] 5) adding 10% trifluoroacetic acid to the product m...
Embodiment 2
[0061] Embodiment 2: Purification and separation by high performance liquid chromatography
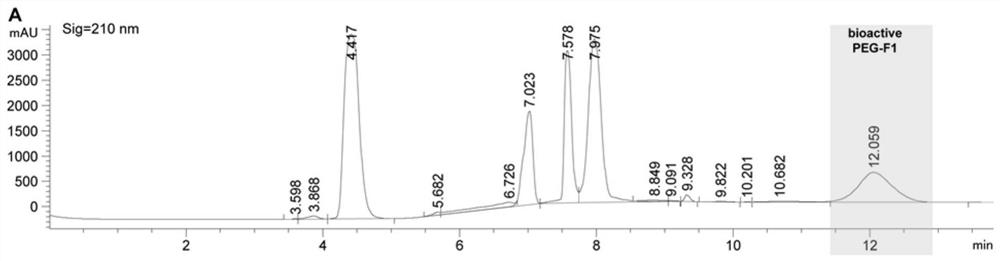
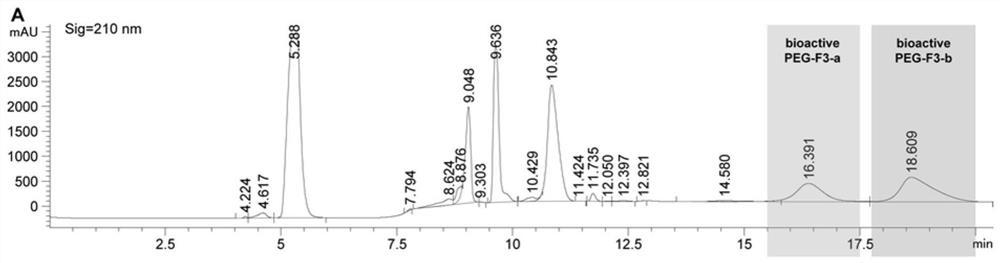
[0062] The PEGylated F polypeptide was subjected to continuous fractionation on an ultra-high performance liquid chromatography (Agilent 1290 Infinity II). The column used is AdvanceBio Peptide Mapping 2.1x150mm, 2.7μm. Two solvents were used: 0.1% trifluoroacetic acid in ultrapure water (A) and 0.1% trifluoroacetic acid in 100% acetonitrile (B), with a flow rate of 0.4 mL / min and a chromatographic signal wavelength of 210 nm. The specific linear gradients are shown in Tables 1 and 2 below. The collected fractions (see figure 1 , 2 ) was lyophilized with a SpeedVac vacuum concentrator (Thermofisher Scientific) and stored at -20°C until use.
[0063] Table 1 Method for separation of PEGylated F1 by high performance liquid chromatography
[0064] Time(min) A(%) B(%) 0.00 97.00 3.00 2.00 97.00 3.00 4.00 34.40 65.60 7.75 32.30 67.70 14.00 32....
Embodiment 3
[0069] Embodiment 3: product structure characterization
[0070] The lyophilized active fraction was redissolved in 50 μL of a mixed solution of 0.1% formic acid + 2% acetonitrile + 98% ultrapure water to make a 0.1 μg / μL peptide solution, and was analyzed by quadrupole time-of-flight mass spectrometry (SCIEX, Concord , Canada) for analysis. Samples were analyzed by LC-MS / MS on an ExionLC liquid chromatography system coupled to a QTOF X500R mass spectrometer (SCIEX, Concord, Canada) equipped with an electrospray ionization source. 15 μL of the sample was injected onto a 100 mm x 1.7 μm Aeris PEPTIDE XB-C18 100 uHPLC column (Phenomenex, Sydney, Australia) equipped with a SecurityGuard column for mass spectrometry. A linear gradient of 5-35% solvent B over 10 min at a flow rate of 400 μL / min, followed by a steeper gradient from 35% to 80% solvent B in 2 min and from 80% to 95% solvent B in 1 min with Eluted with peptide. Solvent B was held at 95% for 1 minute to clean the col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com