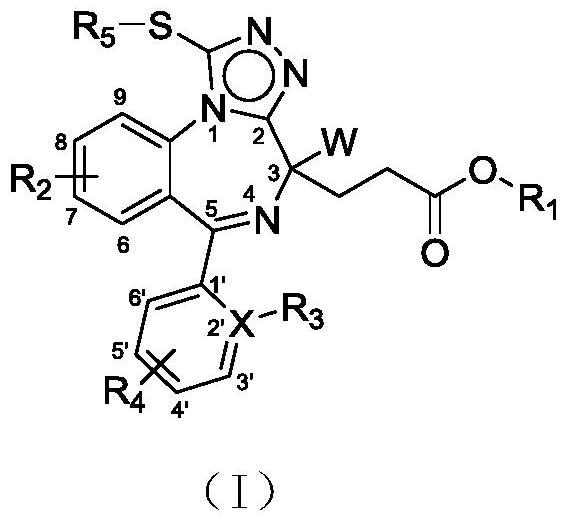
Benzodinitrogen medicine for sedation and hypnosis as well as preparation method and application of benzodinitrogen medicine
A technology of sedation and hypnosis and benzodiazepine, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of reducing nerve excitability, returning patients to normal, drug accumulation, etc., to achieve easy relief Effect of waking up early, reducing side effects, and facilitating metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
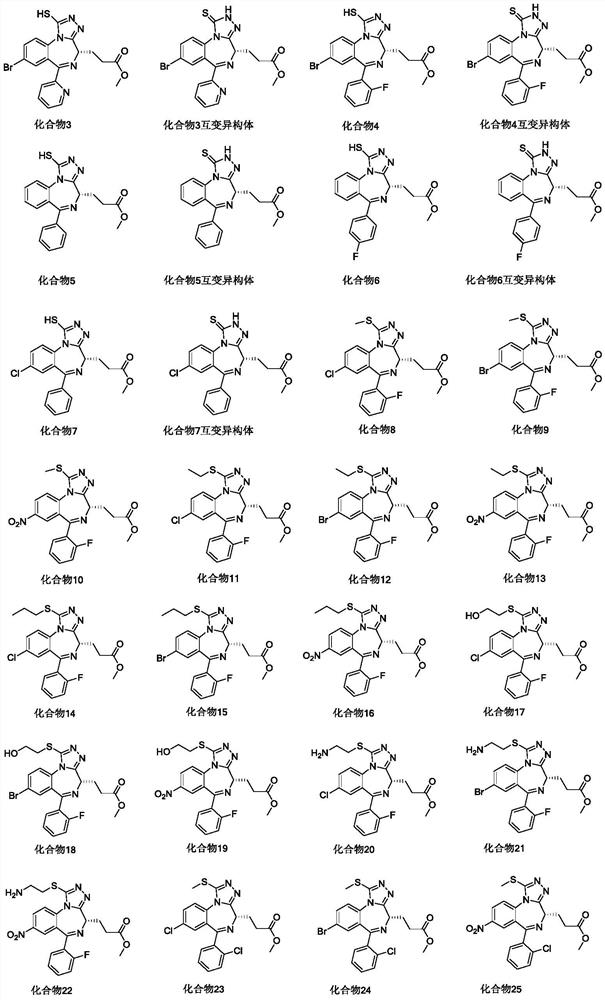
Image
Examples
Embodiment 1
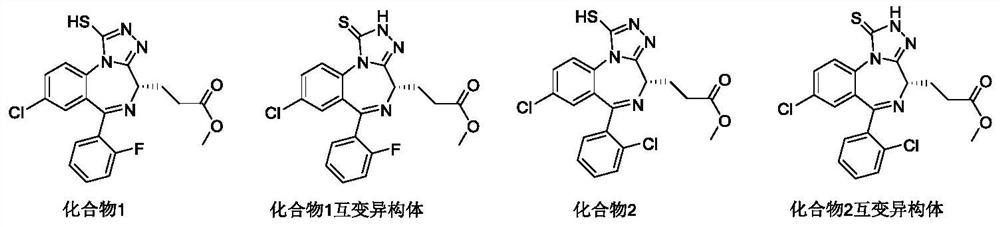
[0199] Example 1: (S)-3-(8-bromo-6-(pyridin-2-yl)-1-thioxo-2-2,4-dihydro-1H-benzo[f][1,2 ,4]triazol[4,3-a][1,4]diazepine -4-yl) methyl propionate (compound 1 tautomer)
[0200]
[0201] The first step: (S)-5-((2-fluoro-benzoyl-4-chlorophenyl) amino)-4-((tert-butoxycarbonyl) amino)-5-oxopentanoic acid methyl ester ( Preparation of Compound 1c)
[0202] 2-Amino-5-chloro-2'-fluorobenzophenone (compound 1a, 11.48g, 0.046mol) and compound N-tert-butoxycarbonyl-L-glutamic acid-5-methyl ester (compound 1b, 10 g, 0.038 mol) was dissolved in DCM (300 mL). The mixture was cooled to 0 °C, DCC (9.49 g, 0.046 mmol) was added, and stirred for 24 hours. LCMS showed the reaction was complete. The filtrate was taken by suction filtration and the reaction solvent was evaporated under reduced pressure, and the residual product was purified by column chromatography (petroleum ether / ethyl acetate, 4:1, v / v) to obtain a white solid (S)-5-((2-fluoro- Benzoyl-4-chlorophenyl)amino)-4-((tert-...
Embodiment 2
[0215] Example 2: (S)-3-(8-chloro-6-(2-chlorophenyl)-1-thio-2,4-dihydro-1H-benzo[f][1,2,4 ]triazol[4,3-a][1,4]diazepine Preparation of -4-yl) methyl propionate (compound 2 tautomer)
[0216] 1 H NMR (300MHz, DMSO-d 6 )δ:14.15(s,1H),8.46(d,J=8.8Hz,1H),7.87(d,J=7.4Hz,1H),7.66-7.50(m,4H),7.16(s,1H), 4.34(t, J=5.8Hz, 1H), 3.62(s, 3H), 2.70-2.45(m, 4H); 13 C NMR (75MHz, DMSO-d 6)δ: 173.54, 167.16, 167.00, 153.68, 138.23, 132.42, 132.28, 132.12, 131.94, 131.73, 131.64, 131.07, 130.21, 128.52, 127.99, 127.966, 55.30, LC / z:447.2[M+H] + .
Embodiment 3
[0217] Example 3: (S)-3-(8-bromo-6-(pyridin-2-yl)-1-thioxo-2,4-dihydro-1H-benzo[f][1,2,4 ]triazol[4,3-a][1,4]diazepine Preparation of -4-yl) methyl propionate (compound 3 tautomer)
[0218] 1 H NMR (300MHz, DMSO-d 6 )δ: 14.17(s, 1H), 8.59(d, J=4.3Hz, 1H), 8.37(d, J=8.8Hz, 1H), 8.13(d, J=7.9Hz, 1H), 8.03-7.98( m,2H),7.60-7.54(m,2H),4.34(t,J=5.8Hz,1H),3.65(s,3H),2.70-2.45(m,4H); 13 C NMR (75MHz, CDCl 3 -d 1 )δ: 173.70, 167.81, 166.51, 157.68, 155.34, 149.03, 136.91, 134.69, 134.01, 132.24, 130.18, 126.91, 125.28, 124.90, 123.99, 55.09, 51.71, 360.18 :457.8[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com