Lysosome fluorescent probe based on chalcone as well as preparation method and application of lysosome fluorescent probe
A technology of fluorescent probes and lysosomes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of cumbersome preparation process, high cost, complex structure, etc., and achieve simple preparation method, low cost, The effect of high degree of matching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method of a chalcone-based lysosome fluorescent probe provided by the invention comprises the following steps:
[0033] Step 1) Add HCA and 1,3-dibromopropane into an organic solvent and mix evenly. The molar ratio of the reaction raw material HCA to 1,3-dibromopropane is 1:2~10. The molar ratio of bromopropane to organic solvent is 1:2~10:60~1000. Under alkaline conditions, heat to 60-120°C, reflux and stir for 4~24h, the reaction raw materials HCA, 1,3-dibromopropane The molar ratio of base to base is 1:2~10:1~10, the solid residue is removed by filtration, the solvent is distilled off under reduced pressure, and the crude product is separated by silica gel column chromatography to obtain the intermediate Br-HCA. The reaction formula is as follows:
[0034]
[0035] Step 2) Add the intermediate Br-HCA and morpholine to the organic solvent and mix evenly, the molar ratio of the intermediate Br-HCA, morpholine and the organic solvent is 1:2~10:60~1000, ...
Embodiment 1
[0038] A preparation method of a lysosome fluorescent probe based on a chalcone molecule, comprising the steps of:
[0039] The preparation of step (1) intermediate Br-HCA
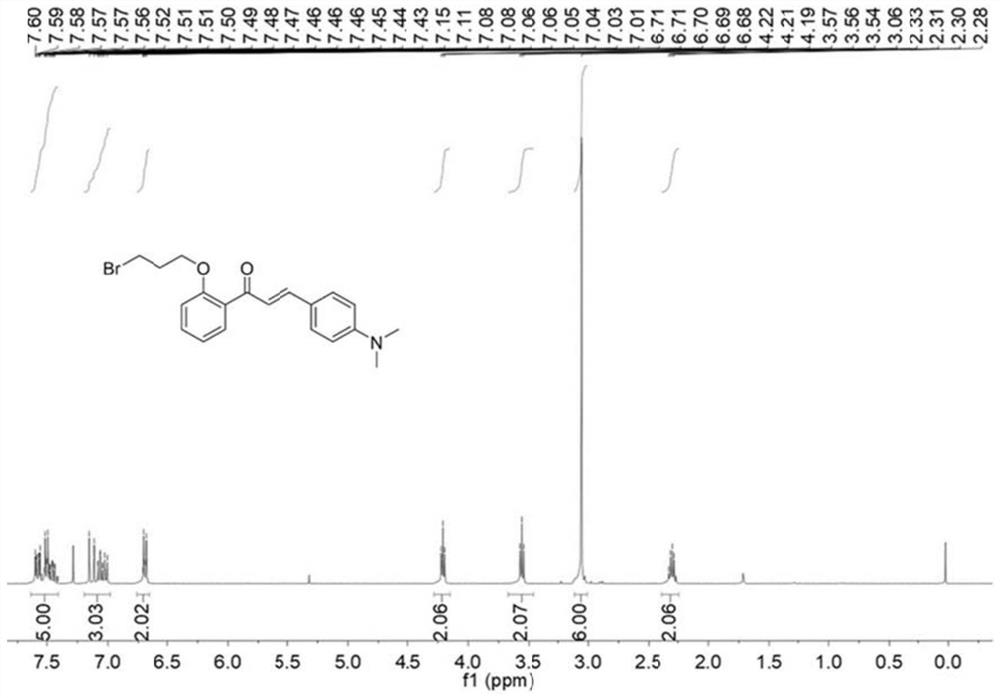
[0040] 2'-Hydroxychalcone (HCA, 0.668g, 2.5mmol) and 1,3-dibromopropane (1.010g, 5mmol) were dissolved in 15mL of N,N-dimethylformamide, and anhydrous potassium carbonate was added (0.518 g, 3.75 mmol). The temperature was raised to 80°C and the reaction was refluxed for 8h. The reaction was stopped, and then extracted with ethyl acetate. After extraction, the organic phases were combined, dried with anhydrous magnesium sulfate, filtered, and distilled under reduced pressure. The crude product was separated by silica gel column chromatography to obtain the compound Br-HCA with a yield of 51%.
[0041] Step (2) Preparation of Lysosome Fluorescent Probe Lyso-HCA
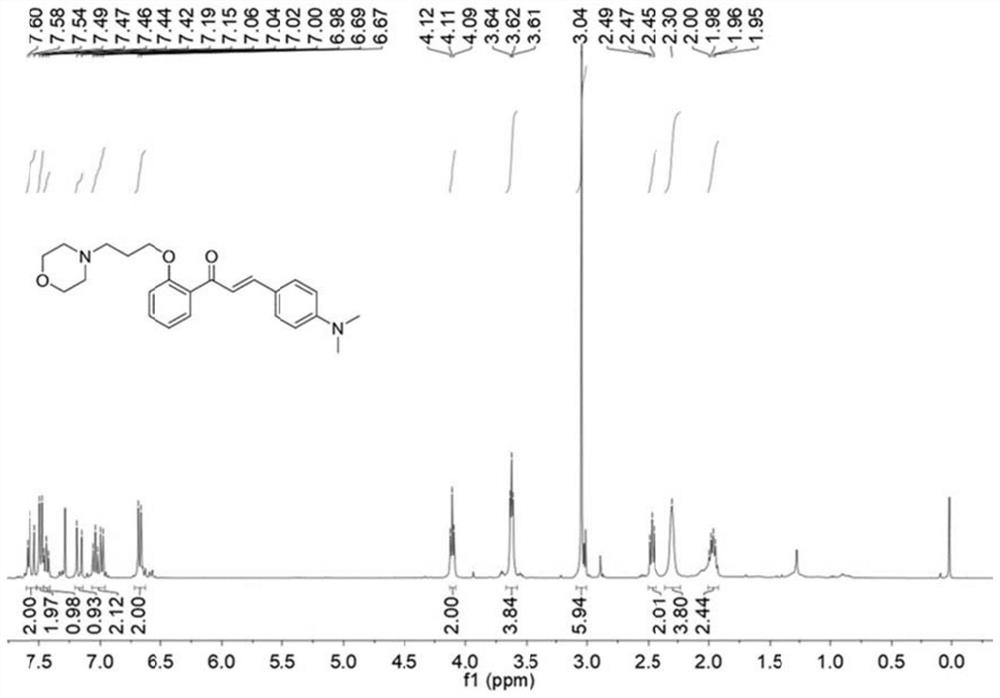
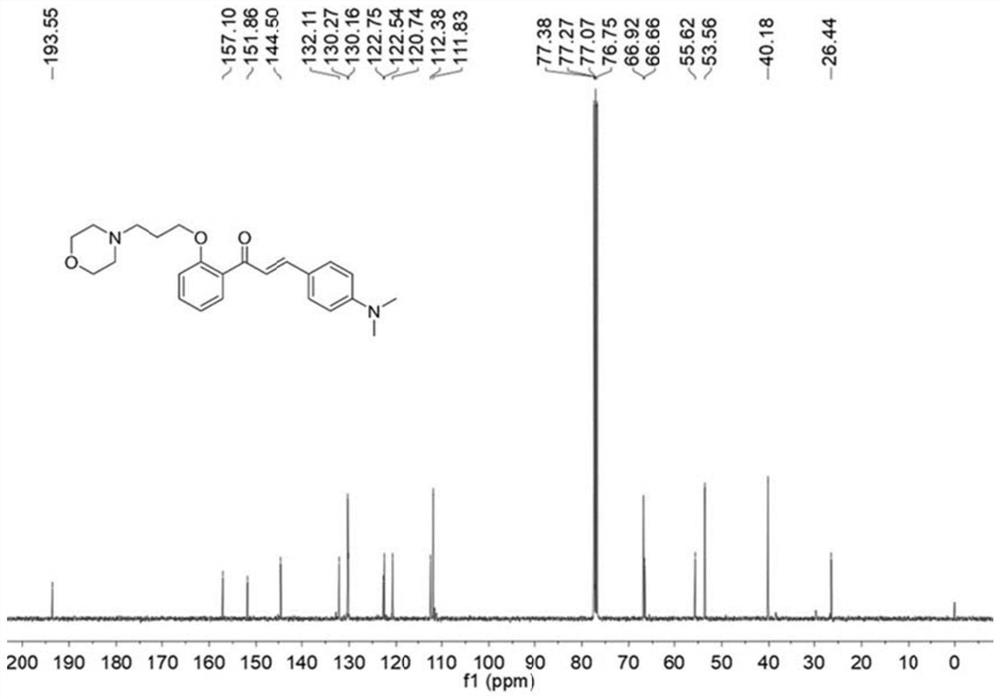
[0042] Compound Br-HCA (39mg, 0.1mmol) and morpholine (43mg, 0.5mmol) were added in a 100mL round bottom flask, and then 5mL of acetonitrile wa...
Embodiment 2
[0044] A preparation method of a lysosome fluorescent probe based on a chalcone molecule, comprising the steps of:
[0045] The preparation of step (1) intermediate Br-HCA
[0046] 2'-Hydroxychalcone HCA (0.267g, 1mmol) and 1,3-dibromopropane (1.010g, 5mmol) were dissolved in 8mL of N,N-dimethylformamide, cesium carbonate (0.652g, 2 mmol). The temperature was raised to 80°C and the reaction was refluxed for 12h. The reaction was stopped, and then extracted with ethyl acetate. After extraction, the organic phases were combined, dried with anhydrous magnesium sulfate, filtered, and distilled under reduced pressure. The crude product was separated by silica gel column chromatography to obtain the compound Br-HCA with a yield of 58%.
[0047] Step (2) Preparation of Lysosome Fluorescent Probe Lyso-HCA
[0048] Compound Br-HCA (78mg, 0.2mmol) and morpholine (70mg, 0.8mmol) were added to a 100mL round bottom flask, then 5mL of acetonitrile was added, cesium carbonate (130mg, 0.4mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com