Magnetic targeting hydrophobic drug carrier hydrogel as well as preparation method and application thereof
A technology for hydrophobic drugs and hydrogels, which can be used in pharmaceutical formulations, liquid delivery, plant raw materials, etc., can solve the problems of short drug release time and the inability of hydrogel decomposition products to be well absorbed by the human body. Wound recovery, universal, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The first aspect of the present invention provides a method for preparing an absorbable drug sustained-release hydrogel carrier, comprising the following steps: adding mono-6-deoxy-ethylenediamine-β-cyclodextrin (β-CD- 6-E) Add collagen to the PBS solution (pH=7.4), stir to dissolve, cool down to 2-8°C, and then mix with 2-8°C 4,5-anhydro-6(n-acetylglucosamine)- Mix the PBS solution (pH=7.4) of oxidized hyaluronic acid (ΔHA-CHO), stir quickly and evenly (about 2-3 minutes), remove the dissolved gas under appropriate pressure, pour it into the mold, and let it stand at room temperature for 24-48 hours. Get hydrogel.
[0032] In some embodiments, the schematic structure of mono-6-deoxy-ethylenediamino-β-cyclodextrin (β-CD-6-E) is as follows:
[0033]
[0034] In some embodiments, the concentration of β-CD-6-E in PBS is 0.6 mg / mL;
[0035] Type I collagen is selected as the collagen, and the primary amino group content of the collagen is 0.508mmol / g;
[0036] The mas...
Embodiment 1
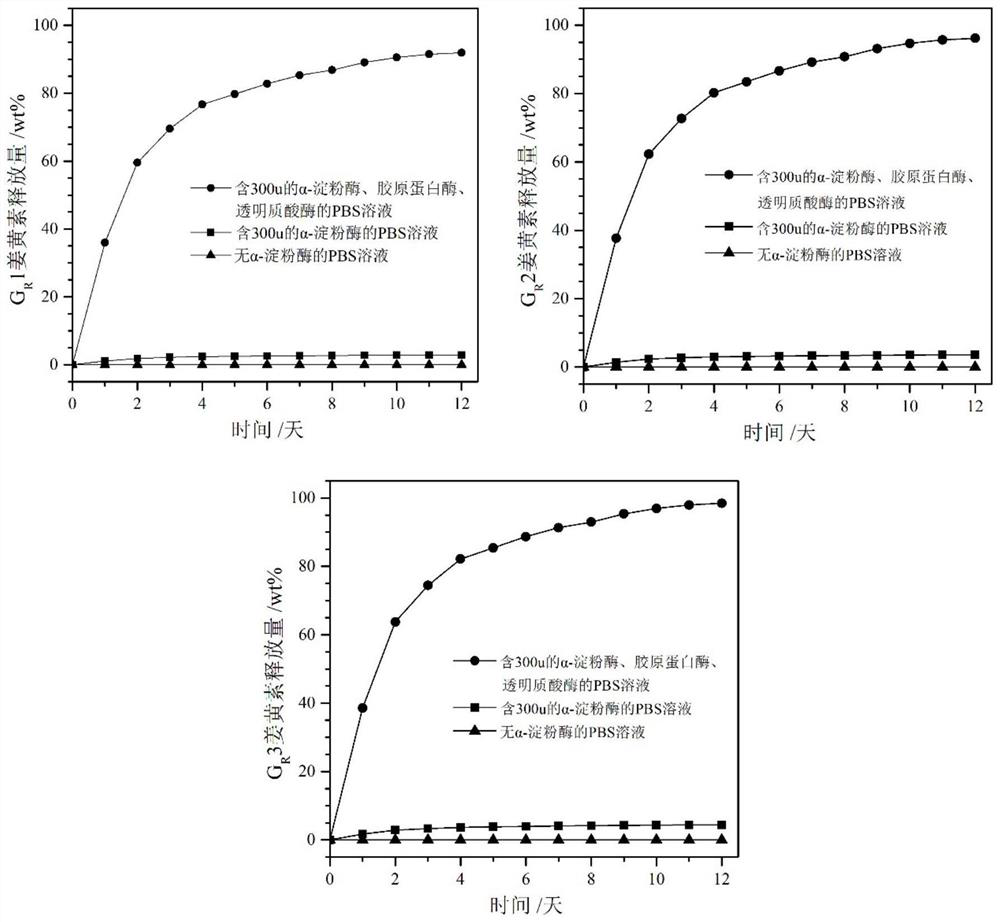
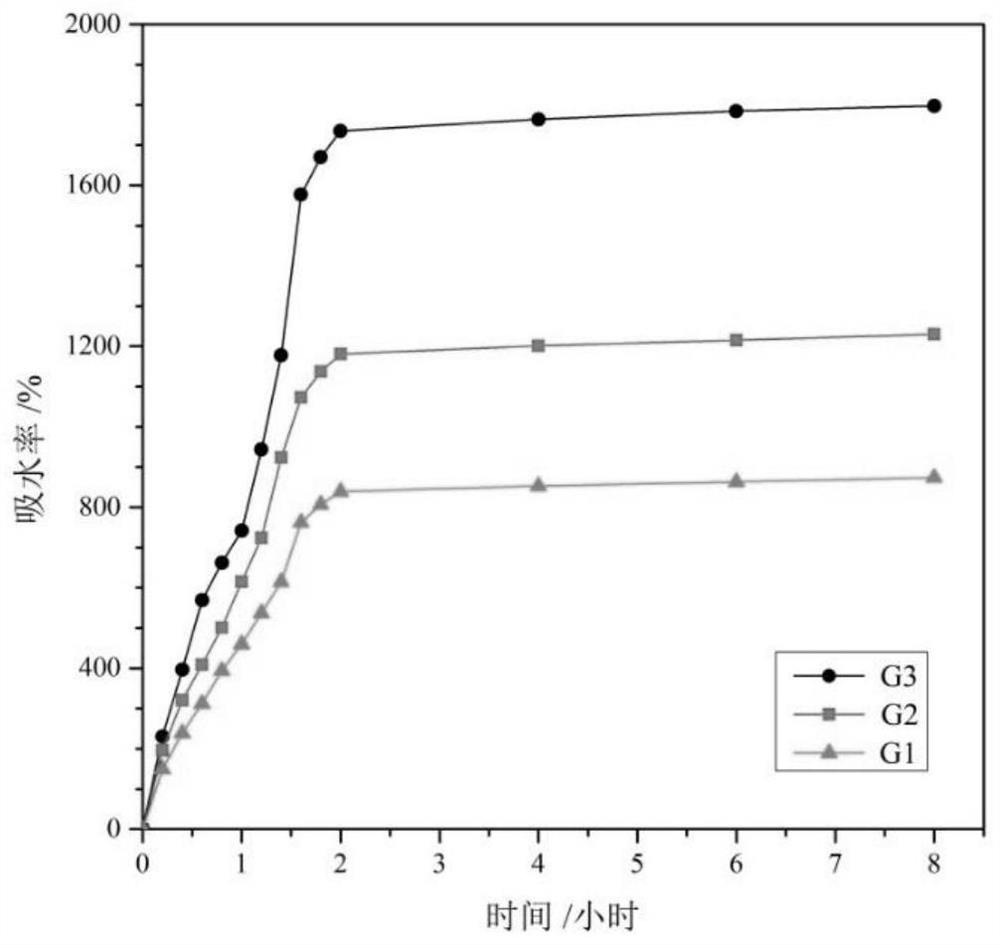
[0054] Preparation of hydrogel: Dissolve 0.06g of mono-6-deoxy-ethylenediamino-β-cyclodextrin in 100mL of PBS solution (pH=7.4), add 1g of collagen, stir to dissolve, cool to 4°C, Then mixed with 4,5-anhydro-6(n-acetylglucosamine)-oxidized hyaluronic acid solution at 4℃ (1.06g 4,5-anhydro-6(n-acetylglucosamine)-oxidized hyaluronic acid Dissolve in 100mL PBS), stir rapidly (about 2-3min), remove dissolved gas under appropriate pressure, pour into a mold, and stand at room temperature for 24-48 hours to obtain hydrogel G1.
[0055] Preparation of drug-loaded hydrogel: Dissolve 0.06g of mono-6-deoxy-ethylenediamino-β-cyclodextrin in 100mL of PBS solution, and add curcumin ethanol solution dropwise under stirring (0.0159g of curcumin in 1mL ethanol), after the dropwise addition, continue to stir for 10 minutes, cool down to 4°C, then add 1g of collagen, stir for 5min, and then mix with 4,5-anhydro-6(n-acetylglucosamine)-oxidized hyaluronic acid at 4°C Acid solution mixing (1.06g ...
Embodiment 2
[0057] Preparation of hydrogel: Dissolve 0.06g of mono-6-deoxy-ethylenediamino-β-cyclodextrin in 100mL of PBS solution (pH=7.4), add 0.8g of collagen, stir to dissolve, and cool down to 4°C , and then mixed with 4,5-anhydro-6(n-acetylglucosamine)-oxidized hyaluronic acid solution at 4°C (0.866g 4,5-anhydro-6(n-acetylglucosamine)-oxidized hyaluronic acid Dissolve acid in 100mL PBS), stir rapidly (about 2-3min), remove dissolved gas under appropriate pressure, pour into a mold, and stand at room temperature for 24-48 hours to obtain hydrogel G2.
[0058] Preparation of drug-loaded hydrogel: Dissolve 0.06g of mono-6-deoxy-ethylenediamino-β-cyclodextrin in 100mL of PBS solution, and add curcumin ethanol solution dropwise under stirring (0.0159g of curcumin in 1mL ethanol), continue stirring for 10 minutes after the dropwise addition, cool down to 4°C, add 0.8g collagen, stir for 5min, and then mix with 4,5-anhydro-6(n-acetylglucosamine)-oxidized transparent Mix the hyaluronic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com