Method for preparing 2, 2 '-dihydroxy-3, 3', 5, 5 '-tetra-tert-butylbiphenyl by using microchannel reactor
A technology of microchannel reactor and di-tert-butylphenol, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of reducing the oxidation reaction rate, unfavorable for scale-up production, difficult separation, etc., to achieve Small footprint, good raw material mixing effect, and good product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
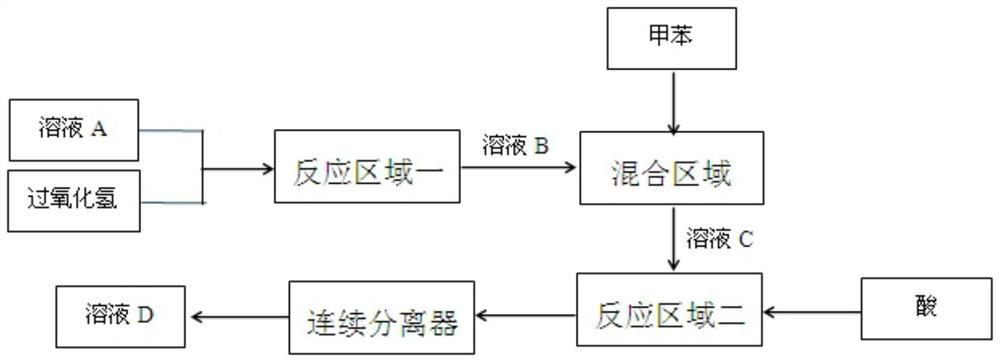
[0033] The adopted microchannel reactor adopts the G1 reaction equipment of Corning Company, uses the metering pump to transport the reaction solution, and sets the module temperature of the first reaction area to 100°C and the module temperature of the second reaction area to 30°C.
[0034] Configuration of solution A: 400 g (10 mol) of sodium hydroxide is dissolved in 2000 g of water, and 1030 g (5 mol) of 2,4-di-tert-butylphenol is added under heating to fully dissolve.
[0035] The flow rate of solution A was 15ml / min, 30% hydrogen peroxide was 15ml / min, toluene was 30ml / min, and sulfuric acid (98%) was pumped into the corresponding reaction module at 2.6ml / min. After the reaction was stabilized, solution D was taken and detected by liquid chromatography: the conversion rate of 2,4-di-tert-butylphenol was 98.6%, and the target product 2,2'-dihydroxy-3,3',5,5'-tetra-tert-butyl The selectivity to biphenyl was 98.2%.
Embodiment 2
[0037] The microchannel reactor used was the G2 reaction equipment of Corning Company, the reaction solution was delivered by a metering pump, and the temperature of the first reaction area module was set to 95°C, and the temperature of the second reaction area module was set to 50°C.
[0038] Configuration of solution A: 400 g (10 mol) of sodium hydroxide is dissolved in 2000 g of water, and 1030 g (5 mol) of 2,4-di-tert-butylphenol is added under heating to fully dissolve.
[0039] The flow rate of solution A is 45ml / min, 30% hydrogen peroxide is 45ml / min, toluene is 90ml / min, and sulfuric acid (98%) is pumped into the corresponding reaction module at 7.8ml / min. After the reaction is stable, solution D is taken and detected by liquid chromatography: the conversion rate of 2,4-di-tert-butylphenol is 98.1%, and the target product 2,2'-dihydroxy-3,3',5,5'-tetra-tert-butyl The selectivity to biphenyl was 98.7%.
Embodiment 3
[0041] The microchannel reactor used was the G1 reaction equipment of Corning Company, and a metering pump was used to transport the reaction solution. The temperature of the first reaction zone module was set to 80°C, and the temperature of the second reaction zone module was set to 50°C.
[0042] Configuration of solution A: 561 g (10 mol) of sodium hydroxide was dissolved in 1839 g of water, and 1030 g (5 mol) of 2,4-di-tert-butylphenol was added under heating until fully dissolved.
[0043]The flow rate of solution A was 15ml / min, 30% hydrogen peroxide was 15ml / min, toluene was 30ml / min, and sulfuric acid (98%) was pumped into the corresponding reaction module at 2.6ml / min. After the reaction was stabilized, solution D was taken and detected by liquid chromatography: the conversion rate of 2,4-di-tert-butylphenol was 95.8%, and the target product 2,2'-dihydroxy-3,3',5,5'-tetra-tert-butyl The selectivity to biphenyl was 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com