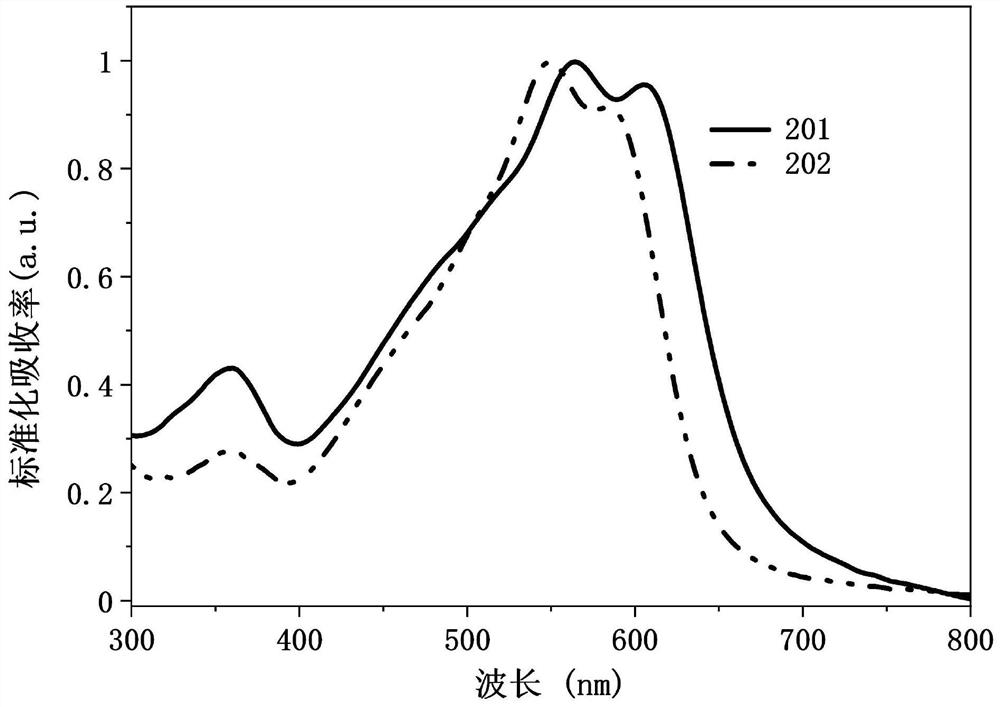
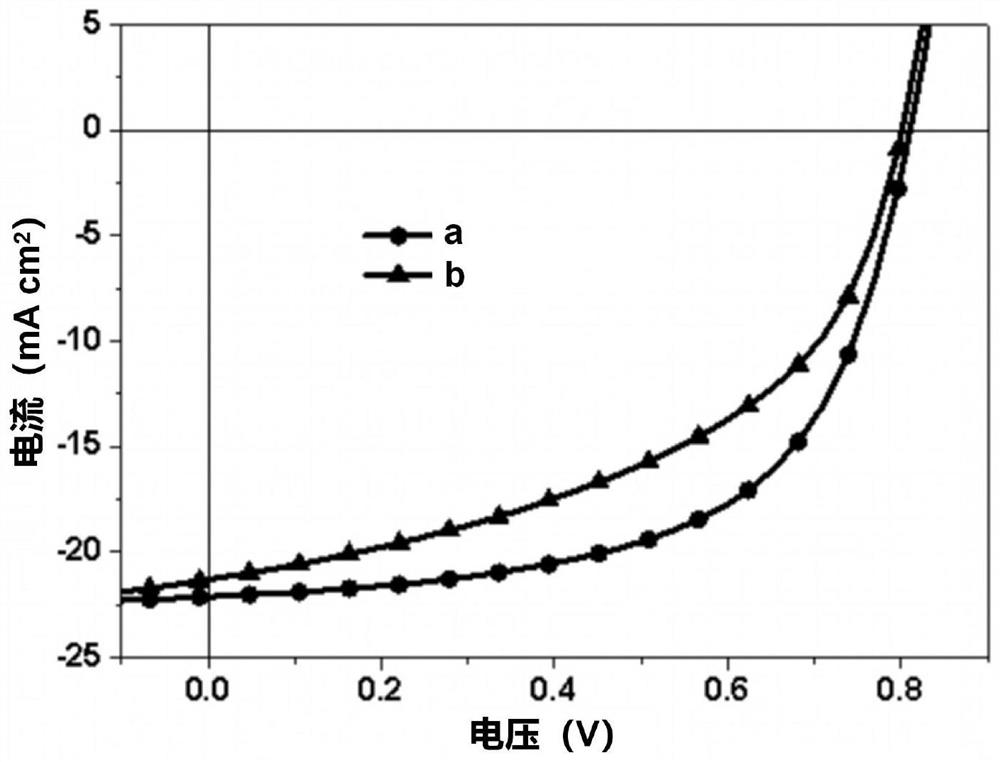
Conjugated organic molecule and preparation method and application thereof
An organic molecule and conjugation technology, applied in the field of solar cells, can solve the problems of complex preparation process of organic solar cells, hindering the commercial application of organic solar cells, and increasing production costs, achieving broad commercial application prospects, low production costs, and high-tech The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The present invention also provides the preparation method of the above-mentioned conjugated organic molecule, comprising:
[0059] S11, dissolving the first precursor and the second precursor in the first solvent, and reacting to obtain the third precursor;
[0060] S12, dissolving the third precursor and the precursor containing the electron withdrawing unit in the second solvent, and reacting to obtain a conjugated organic molecule.
[0061] In step S11, the first precursor includes at least a compound represented by the following formula (8),
[0062]
[0063] where R 9 , R 10 , R 11 Independently selected from at least one of straight-chain alkyl groups having 1-10 carbon atoms and branched-chain alkyl groups having 2-10 carbon atoms;
[0064] The first precursor is preferably a compound represented by the following formula (8-1),
[0065]
[0066] The function of the first precursor is to provide terphenyl dithiophene as the central unit of the conjugat...
Embodiment 1
[0112] The conjugated organic molecule 3BDT-4 was prepared by the following procedure:
[0113]
[0114] In the three-necked flask, add compound 8-1 (1.03 g, 0.50 mmol), compound 9-1 (0.41 g, 1.05 mmol), and 70 mL of anhydrous toluene, feed N 2 Deoxygenation was carried out for 30 min, then tetrakistriphenylphosphonium palladium (60 mg) was added, and the reaction was refluxed for about 24 h. After the reaction was completed, it was concentrated to dryness under reduced pressure, and subjected to column chromatography (eluent: petroleum ether (PE): chloroform (CHCl) 3 )=3:1) separation and purification to obtain a dark purple solid compound 10-10.60 g with a yield of 51%. HNMR test data: H NMR (400MHz, CDCl 3 ,ppm)δ:9.75(s,2H,-CHO),7.58(d,2H,J=2.8Hz,ArH),7.42-7.38(m,6H,ArH),7.27-7.25(m,6H,ArH) ,7.05(d,2H,J=3.2Hz,ArH),6.92(d,6H,J=2.8Hz,ArH),6.82(s,2H,ArH),2.95(d,12H,J=6.4Hz,- CH 2 -),2.63(t,4H,J=7.2Hz,-CH 2 -),1.84-1.76(m,6H,-CH-),1.62-1.29(m,72H,-CH-) 2 -),1.06-0.88(m,...
Embodiment 2
[0117] The conjugated organic molecule 3BDT-5 was prepared by the following scheme:
[0118]
[0119] The preparation method of compound 10-1 is the same as that in Example 1. Next, in a three-necked flask, add compound 10-1 (0.30 g, 0.128 mmol), compound 13-1 (0.25 g, 1.28 mmol) and 60 mL of chloroform, and 0.1 mL of piperidine, and react at 60 ° C for 24 h under nitrogen protection . After the reaction, it was poured into 200 mL of methanol for sedimentation, and the obtained solid was separated and purified by column chromatography (eluent: PE:CHCl). 3 = 1:2), the crude product is obtained. Using methanol:chloroform (volume ratio 1:2) as a mixed solvent, recrystallization was performed twice, and finally a dark red solid 3BDT-50.28g was obtained with a yield of 82%. The HNMR test data are as follows: HNMR (400MHz, CDCl 3 ,ppm)δ:8.03(s,2H,ArH),7.59(s,2H,ArH),7.36-7.25(m,12H,ArH),7.03(s,2H,ArH),6.93-6.91(m,6H ,ArH),6.82(s,2H,ArH),4.12(br,4H,-OCH 2 -),2.97(d,12H,J=6.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com