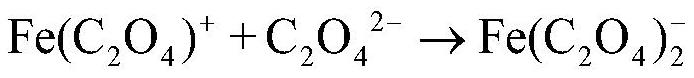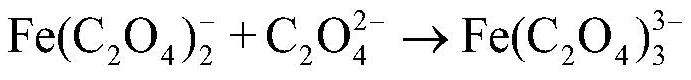Method for preparing ferrous oxalate from siderite
A technology of ferrous oxalate and siderite, which is applied in carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of high energy consumption and low purity of ferrous oxalate, and achieve a high level of clean production , high purity, and remarkable environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
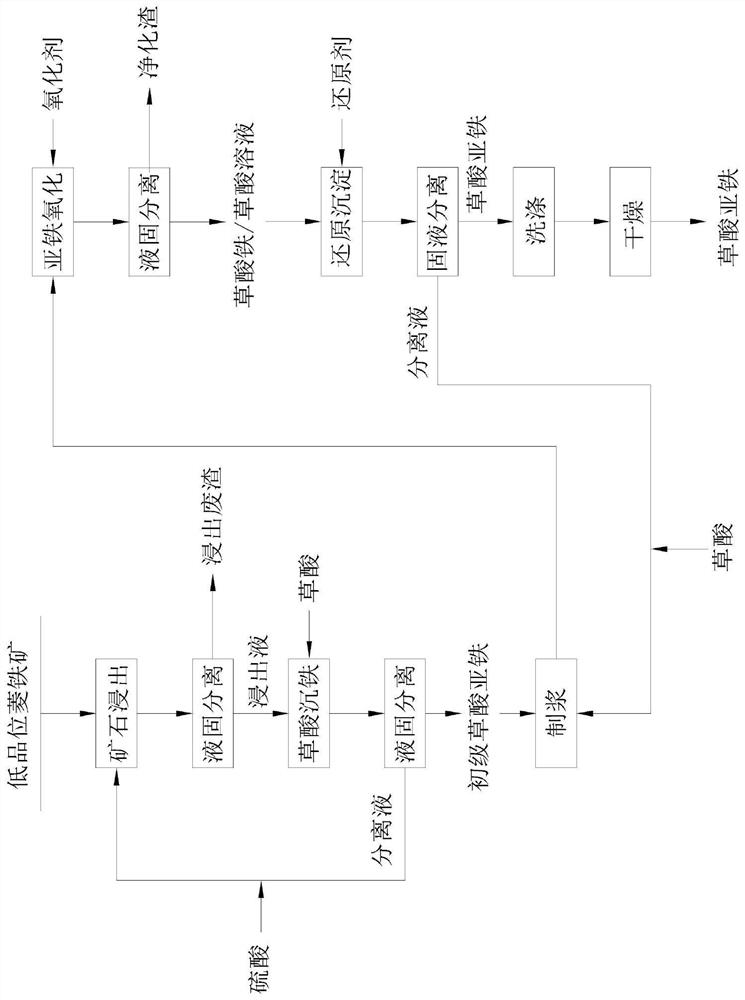
[0058] A method utilizing siderite to prepare ferrous oxalate, comprising the following steps:
[0059] (1) Ore leaching: Grind 100kg of low-grade siderite into fine particles with a particle size of 100 mesh, add it to dilute sulfuric acid solution, and conduct a leaching reaction at 80°C for 4 hours to convert the iron carbonate in the siderite into soluble ferrous ions; wherein, the liquid-solid ratio of dilute sulfuric acid solution and siderite is 8:1, and the molar ratio of iron in sulfuric acid and siderite is 1.3:1;
[0060] (2) Liquid-solid separation: the slurry obtained in step (1) is subjected to pressure filtration to obtain slag and a separating liquid containing ferrous sulfate;
[0061] (3) Precipitating iron oxalate (removing potassium and sodium): add oxalic acid to the separation liquid 1, carry out precipitation reaction for 0.5h at 30°C, and then separate solid and liquid to obtain primary ferrous oxalate and separation liquid 2; among them, oxalic acid T...
Embodiment 2
[0070] A method utilizing siderite to prepare ferrous oxalate, comprising the following steps:
[0071] (1) Ore leaching: Grind 100kg of low-grade siderite into fine particles with a particle size of 100 mesh, add it to dilute sulfuric acid solution, and conduct a leaching reaction at 70°C for 5 hours to convert the iron carbonate in the siderite into soluble ferrous ions; wherein, the liquid-solid ratio of dilute sulfuric acid solution and siderite is 1:1, and the molar ratio of iron in sulfuric acid and siderite is 1.1:1;
[0072] (2) Liquid-solid separation: the slurry obtained in step (1) is subjected to pressure filtration to obtain slag and a separating liquid containing ferrous sulfate;
[0073] (3) Precipitating iron oxalate (removing potassium and sodium): add oxalic acid to the separation liquid 1, carry out precipitation reaction at 30°C for 0.6h, and then separate solid and liquid to obtain primary ferrous oxalate and separation liquid 2; among them, oxalic acid T...
Embodiment 3
[0082] A method utilizing siderite to prepare ferrous oxalate, comprising the following steps:
[0083] (1) Ore leaching: Grind 100kg of low-grade siderite into fine particles with a particle size of 100 mesh, add it to dilute sulfuric acid solution, and conduct a leaching reaction at 100°C for 3 hours to convert the iron carbonate in the siderite into soluble ferrous ions; wherein, the liquid-solid ratio of dilute sulfuric acid solution and siderite is 10:1, and the molar ratio of iron in sulfuric acid and siderite is 1.5:1;
[0084] (2) Liquid-solid separation: the slurry obtained in step (1) is subjected to pressure filtration to obtain slag and a separating liquid containing ferrous sulfate;
[0085] (3) Precipitating iron oxalate (removing potassium and sodium): add oxalic acid to the separation liquid 1, carry out precipitation reaction for 0.5h at 30°C, and then separate solid and liquid to obtain primary ferrous oxalate and separation liquid 2; among them, oxalic acid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com