Infection enhancing culture medium and method for improving cell lentivirus infection rate
A medium and infection rate technology, applied in the direction of cell culture active agent, virus, culture process, etc., can solve the problems of gene editing efficiency constraints, low knockout positive rate, poor cell state, etc., to increase the infection rate of cell lentivirus , Improve the culture and infection rate, reduce the effect of operation difficulty and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
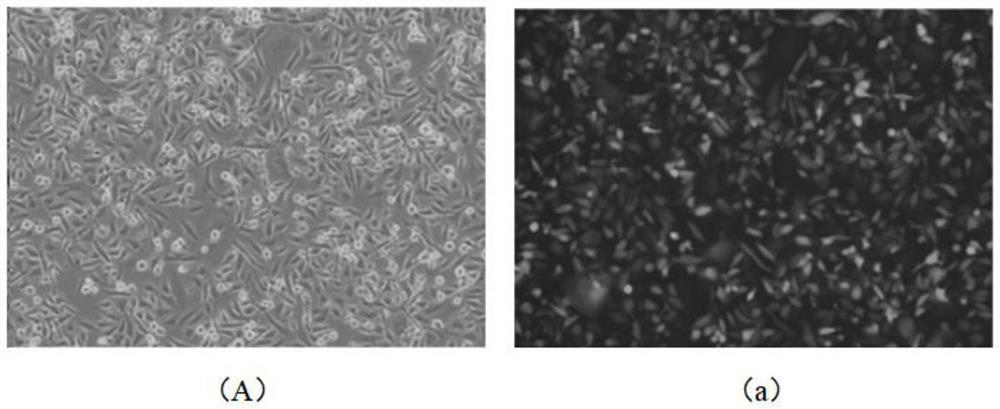
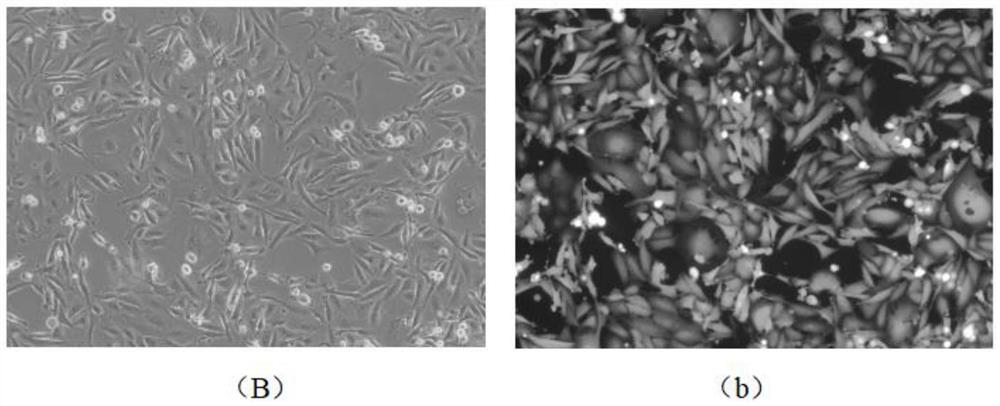
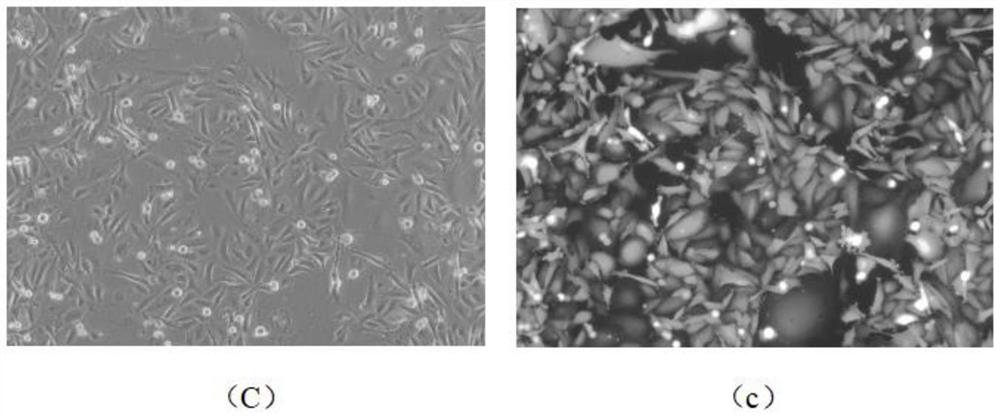
[0072] A method for improving the infection rate of MDA-MB-231 cells, comprising the following steps;
[0073] (1) Digest the MDA-MB-231 cells in the logarithmic growth phase into a suspension single-cell liquid, and prepare 1×10 5 Amount of cell inoculation per well Inoculate the single cell suspension into a 12-well cell culture plate, add 1ml of conventional medium, and culture at 37°C and ambient air, wherein the conventional medium contains glutamine and Leibovitz's L-15 medium with fetal bovine serum.
[0074] (2) When the MDA-MB-231 cells in step (1) are cultured for 36 hours, replace the conventional culture medium with enhanced infection medium, and cultivate them at 37°C and in an environment with a volume fraction of 5% carbon dioxide for 24 hours , wherein the components of the enhanced infection medium are shown in Table 1 below;
[0075] (3) Maintain the culture conditions of step (2), use the lentivirus with EGFP expression box to infect MDA-MB-231 cells, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com