Method for preparing bromoxynil octanoate
A technology for octanoyl bromoxynil and bromoxynil, which is applied in the field of octanoyl bromoxynil synthesis, can solve the problems of high price of octanoyl chloride, environmental pollution and high reaction temperature, and achieves enhanced mass transfer effect, shortened reaction time, and reduced reaction time. fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
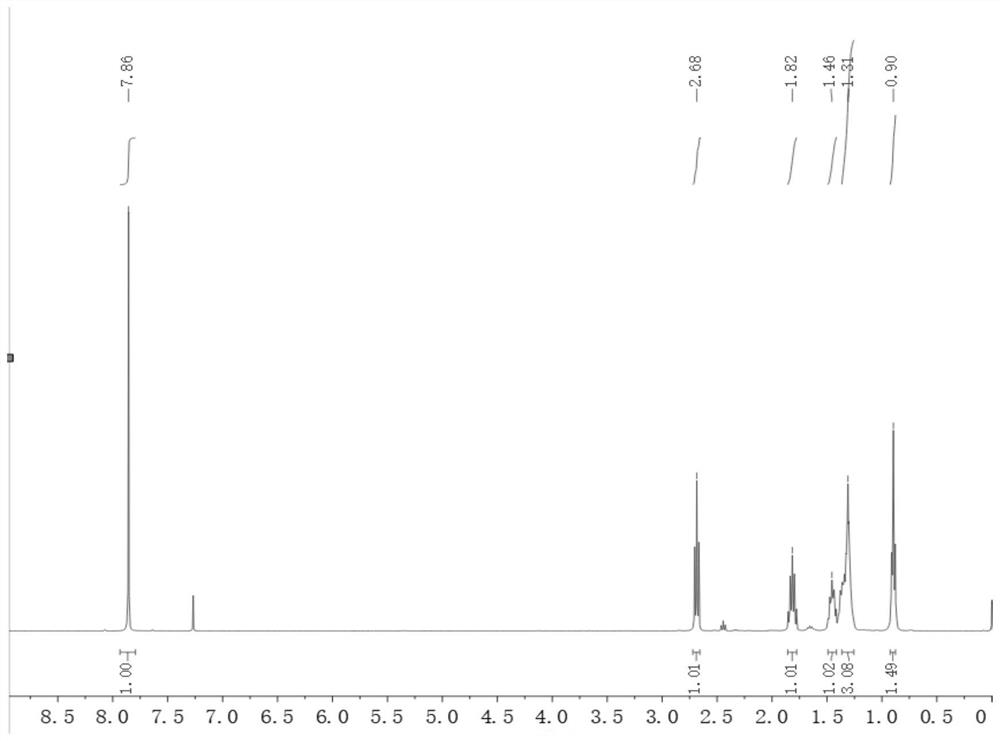
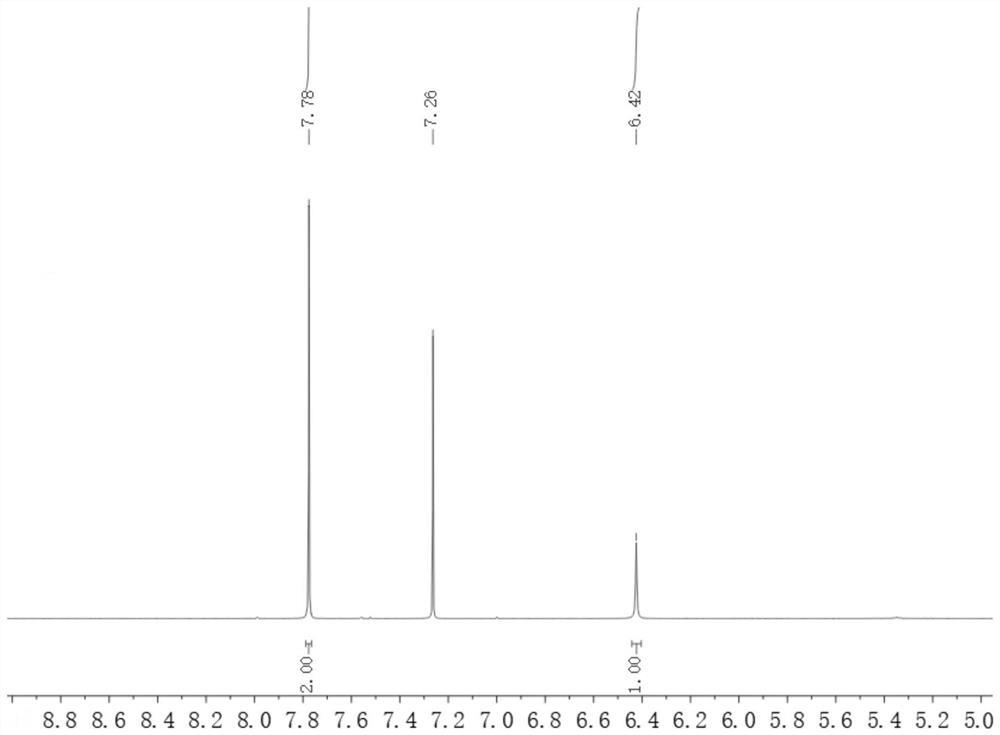
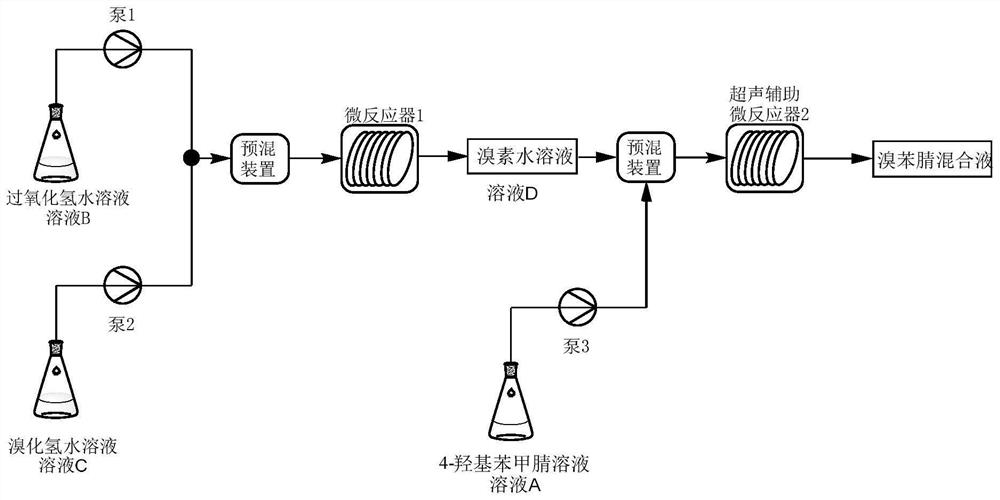
[0042]Take 48g (0.4mol) of 4-hydroxybenzonitrile, add 480g of absolute ethanol and mix to obtain a homogeneous organic solution; take 297g (1.76mol) of 48% aqueous hydrobromic acid solution; take 100g (0.88mol) of 30% peroxide oxidation Hydrogen aqueous solution; pour the prepared three materials into the corresponding raw material tanks respectively, set the temperature of the microreactor 1 to 5 °C, open the ultrasonic-assisted (frequency 35Hz) microreactor 2, and set the temperature of the ultrasonic device to 50 °C. °C. The hydrobromic acid aqueous solution and the hydrogen peroxide aqueous solution were pumped into the microreactor 1, wherein the hydrobromic acid aqueous solution was pumped in at a flow rate of 4 mL / min, and the hydrogen peroxide aqueous solution was pumped in at a flow rate of 1.8 mL / min. The reaction temperature was 5°C, and the reaction residence time was 5 min to obtain an aqueous solution of bromine. The 4-hydroxybenzonitrile solution and the bromin...
Embodiment 2
[0043] Example 2: Comparative Example 1, hydrobromic acid reduced by 25%.
[0044] Take 48g (0.4mol) of 4-hydroxybenzonitrile, add 480g of absolute ethanol and mix to obtain a homogeneous organic solution; take 223g (1.32mol) of 48% aqueous hydrobromic acid solution; take 100g (0.88mol) of 30% peroxide oxidation Hydrogen aqueous solution; pour the prepared three materials into the corresponding raw material tanks respectively, set the temperature of the microreactor 1 to 5 °C, open the ultrasonic-assisted (frequency 35Hz) microreactor 2, and set the temperature of the ultrasonic device to 50 °C. °C. The hydrobromic acid aqueous solution and the hydrogen peroxide aqueous solution were pumped into the microreactor 1, wherein the hydrobromic acid aqueous solution was pumped in at a flow rate of 4 mL / min, and the hydrogen peroxide aqueous solution was pumped in at a flow rate of 1.8 mL / min. The reaction temperature was 5°C, and the reaction residence time was 5 min to obtain an a...
Embodiment 3
[0045] Example 3: Comparative Example 1, without ultrasonic assistance
[0046] Take 50.88g (0.424mol) of 4-hydroxybenzonitrile, add 508g of absolute ethanol and mix to obtain a homogeneous organic solution; take 315g (1.87mol) of 48% aqueous hydrobromic acid; take 106g (0.93mol) of 30% peroxide Hydrogen oxide aqueous solution; pour the prepared three materials into the corresponding raw material tanks respectively, set the temperature of the microreactor 1 to 5°C, open the ultrasonic-assisted (frequency 35Hz) microreactor 2, and set the temperature of the ultrasonic device to 50°C. The hydrobromic acid aqueous solution and the hydrogen peroxide aqueous solution were pumped into the microreactor 1, wherein the hydrobromic acid aqueous solution was pumped in at a flow rate of 4 mL / min, and the hydrogen peroxide aqueous solution was pumped in at a flow rate of 1.8 mL / min. The reaction temperature was 5°C, and the reaction residence time was 5 min to obtain an aqueous solution o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com