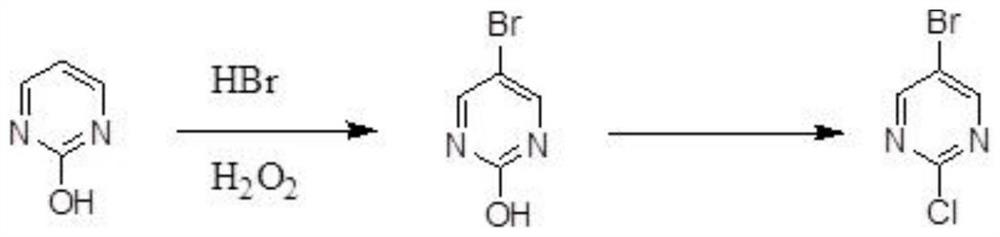
One-step synthesis method of 5-bromo-2-chloropyrimidine
A synthesis method and chloropyrimidine technology are applied in the field of one-step synthesis method of 5-bromo-2-chloropyrimidine, which can solve the problems of low utilization rate of raw materials, low efficiency, human body pollution and the like, and achieves simplified production process, improved production efficiency, The effect of improving economic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A one-step synthesis method of 5-bromo-2-chloropyrimidine, the method is:
[0049] Take 112.1g (1mol) of 2-hydroxypyrimidine, mix 2-hydroxypyrimidine with 404.6g of hydrobromic acid with a concentration of 20wt%, the molar ratio of hydrogen bromide and 2-hydroxypyrimidine in the hydrobromic acid is 1:1, add 340 g of hydrogen peroxide with a concentration of 10wt%, the molar ratio of hydrogen peroxide and 2-hydroxypyrimidine in the hydrogen peroxide is 1:1; heated to 100°C in a reaction vessel for 8h insulation reaction to obtain 5-bromo-2-hydroxypyrimidine;
[0050] Add catalase in the proportion of 150IU / mL reaction solution, react for 30min, then cool down to ≤10℃, filter under reduced pressure to remove the filtrate, and blow dry at 75℃ for 5h;
[0051] Subsequently, 153.3g (1mol) of phosphorus oxychloride and 60.7g (0.6mol) of triethylamine were added to the reaction vessel, and the reaction vessel was heated to 120° C. for 8 hours, and the preparation of 5-bromo-2-...
Embodiment 2
[0057] A one-step synthesis method of 5-bromo-2-chloropyrimidine, the method is:
[0058] Take 112.1g (1mol) of 2-hydroxypyrimidine, mix 2-hydroxypyrimidine with 462.3g of hydrobromic acid with a concentration of 35wt%, the molar ratio of hydrogen bromide and 2-hydroxypyrimidine in the hydrobromic acid is 2:1, add 226.7g of hydrogen peroxide with a concentration of 30wt%, the molar ratio of hydrogen peroxide and 2-hydroxypyrimidine in the hydrogen peroxide is 2:1; heated to 40°C in a reaction vessel for 12 hours of insulation reaction to obtain 5-bromo-2-hydroxypyrimidine;
[0059] Catalase was added in the proportion of 150IU / mL reaction solution, reacted for 30min, cooled to ≤10℃, filtered under reduced pressure to remove the filtrate, and air-dried at 85℃ for 4h;
[0060] Subsequently, 191.7g (1.25mol) of phosphorus oxychloride and 65.8g (0.65mol) of triethylamine were added to the reaction vessel, and the reaction vessel was heated to 80°C for 6 hours, and the reaction was...
Embodiment 3
[0064] A one-step synthesis method of 5-bromo-2-chloropyrimidine, the method is:
[0065] Take 112.1g (1mol) of 2-hydroxypyrimidine, mix 2-hydroxypyrimidine with 485.5g of hydrobromic acid with a concentration of 50wt%, the molar ratio of hydrogen bromide and 2-hydroxypyrimidine in the hydrobromic acid is 3:1, add 340 g of hydrogen peroxide with a concentration of 50wt%, the molar ratio of hydrogen peroxide and 2-hydroxypyrimidine in the hydrogen peroxide is 5:1; heated to 30°C in a reaction vessel for 14 hours of insulation reaction to obtain 5-bromo-2-hydroxypyrimidine;
[0066] Add catalase in the proportion of 150IU / mL reaction solution, react for 30min and then cool down to ≤10℃, filter under reduced pressure to remove the filtrate, and blow dry at 70℃ for 6h;
[0067] Subsequently, 766.7g (5mol) of phosphorus oxychloride and 202.4g (2mol) of triethylamine were added to the reaction vessel, and the reaction vessel was heated to 50°C for 5h and the reaction was maintained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com