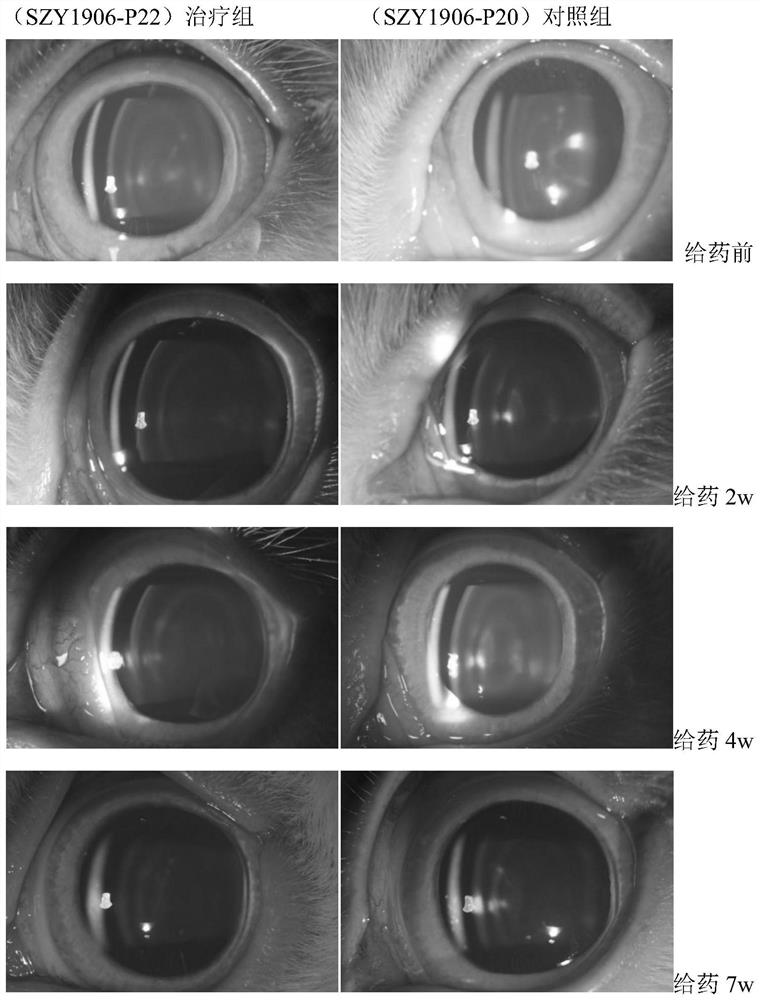
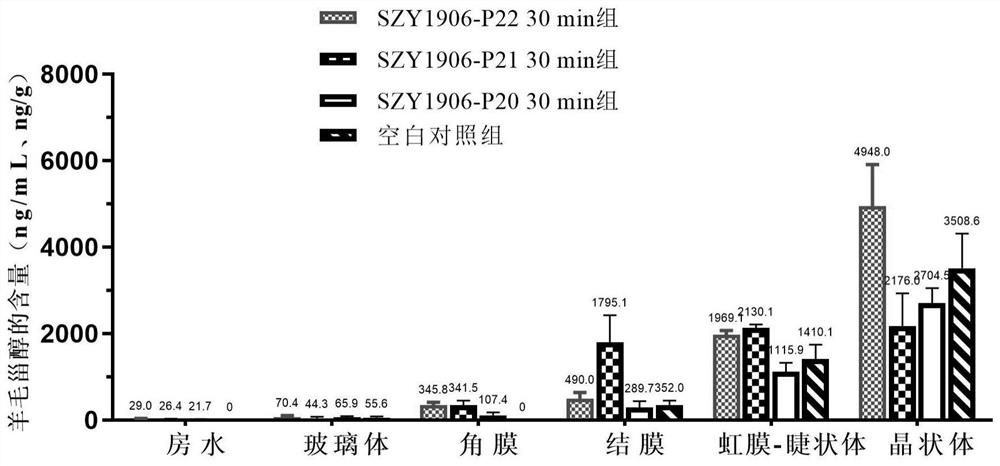
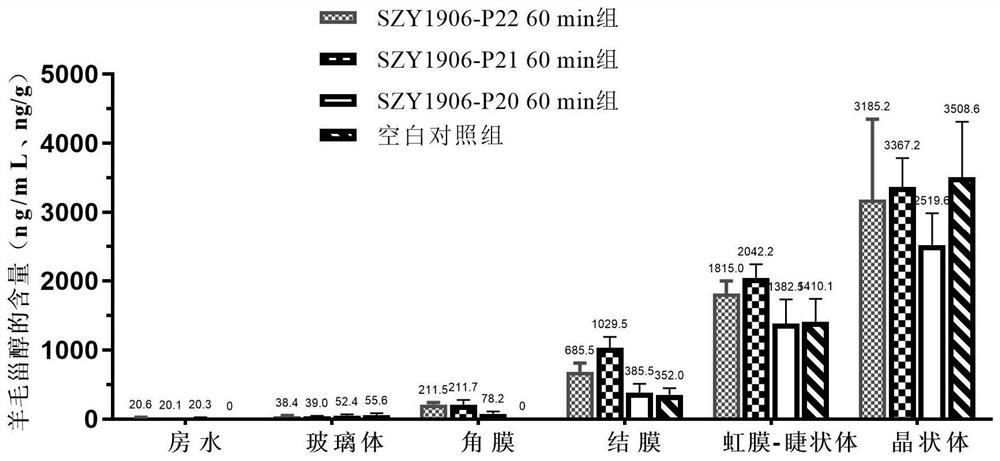
Steroid compound as well as preparation method and application thereof
A compound and composition technology, applied in the field of medicine, can solve problems such as poor drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Synthesis of compound I-1 (SZY1906-P21)
[0044] Specific steps are as follows:
[0045] 1.1. Synthesis of CDP-Y120-3:
[0046]
[0047] Add CDP-Y120-1 (25g) to a 1000ml round-bottomed flask, add 500ml DMF and stir to form a suspension, add imidazole (9.56g), stir and react for half an hour, add TBSCl (10.6g) in batches, heat to 80°C Dissolved and reacted for 8h. The temperature of the reaction solution was monitored by TLC until the reaction was completed, and the temperature of the reaction solution was lowered to room temperature. The reaction solution was added to 3000ml of water, extracted with DCM 500ml*5, the organic phases were combined and concentrated, washed with 3000ml of water again, extracted with DCM 500ml*5, and the organic phases were combined and concentrated. Repeat the operation one more time. The organic phase was dried with anhydrous sodium sulfate, rotary evaporated, and purified by column chromatography four times using n-hexane ...
Embodiment 2
[0056] Example 2. Synthesis of compound I-2 (SZY1906-P23)
[0057]
[0058] Add CDP-Y120-1 (2.8g) to a 100ml round-bottomed flask, add 80ml DCM to dissolve, add DMAP (160mg) and stir for half an hour, then add EDCI (2.5g), o-methylaminobenzoic acid (1.2g), The reaction was stirred for 12h. The reaction solution was washed with 700 ml of water, extracted with DCM 300*3, dried and rotary evaporated, and purified by column chromatography to obtain about 0.85 g of white solid compound I-2.
[0059] 1 HNMR CDCl 3 δ:7.64-7.70(d,J=8.0Hz,1H),7.32-7.38(t,1H),6.94-6.98(d,J=8.0Hz,1H),6.83-6.88(t,1H),5.11- 5.17(t,1H), 4.70-4.76(q,1H), 3.72-3.79(q,1H), 2.85-2.95(s,3H), 2.00-2.14(q,5H), 1.72-1.90(q,10H) ),1.58-1.66(q,8H),1.27-1.47(q,6H),0.90-1.06(q,16H),0.72(s,3H)
[0060] LC-MS: m / z=574.4 (M+1).
Embodiment 3
[0061] Example 3. Synthesis of compound I-3 (SZY1906-P22)
[0062]
[0063] Add CDP-Y120-1 (2.2g) to a 100ml round bottom flask, add 80ml DCM to dissolve, add DMAP (125mg) and stir for half an hour, then add EDCI (1.98g), o-dimethylaminobenzoic acid (1.02g) , and the reaction was stirred for 12h. The reaction solution was washed with 500 ml of water, extracted with 300 ml of DCM*3, dried and rotary evaporated, and purified by column chromatography to obtain about 1.9 g of white solid compound I-3.
[0064] 1HNMR CDCl 3 δ:7.66-7.72(d,J=8.0Hz,1H),7.33-7.39(t,1H),6.96-7.00(d,J=8.0Hz,1H),6.84-6.89(t,1H),5.10- 5.16(t, 1H), 4.72-4.78(q, 1H), 3.71-3.79(q, 1H), 2.83-2.93(s, 6H), 2.01-2.11(t, 5H), 1.72-1.92(t, 10H) ),1.56-1.64(t,8H),1.25-1.47(t,6H),0.90-1.04(t,16H),0.72(s,3H)
[0065] LC-MS: m / z=588.5 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com