Near-infrared IIb fluorescent probe, nano particle and preparation method and application thereof
A technology of fluorescent probes and nanoparticles, applied in the field of bio-optical diagnosis and treatment, can solve the problems of long-term biological toxicity limiting clinical practical application, and achieve the effects of low toxicity, good repeatability, and high fluorescence brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A NIR-IIb fluorescent probe based on diketopyrrolopyrrole (DPP) conjugated polymer, wherein the conjugated polymer DT-H has the following structure:
[0033]
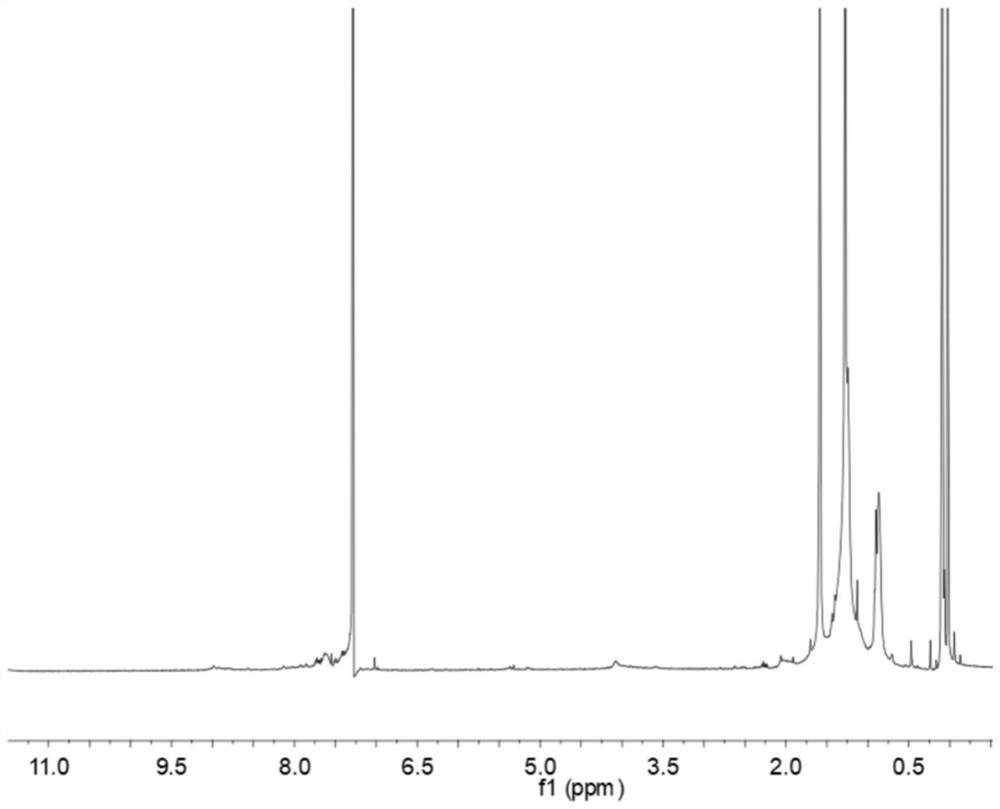
[0034] The synthesis steps of the above-mentioned conjugated polymer DT-H include: 2,5-bis(2-octyldodecyl)-3,6-bis(5-(trimethyltin)thiophene-2-yl) -2,5-Dihydropyrrolo[3,4-c]pyrrole-1,4-dione (0.05 g, 0.042 mmol), 4,7-bis(5-bromothiophen-2-yl)benzo[ c][1,2,5]thiadiazole (0.0193 g, 0.042 mmol), Pd (PPh 3 ) 4 (5 mg, 0.0042 mmol) and 10 mL of toluene in N 2 It was added to a 50 mL preset reaction tube under the atmosphere, and the mixture was refluxed for 7 h. After cooling to room temperature, the reaction solution was added dropwise to 200 mL methanol solution, and the mixture was collected by filtration under reduced pressure to obtain the final product DT-H (the yield was 58%). ), and its H NMR spectrum is shown in figure 1 As shown, the peak between 6.5-8.0 in the figure is the hydrogen on the aromatic ri...
Embodiment 2
[0041] A NIR-IIb fluorescent probe based on diketopyrrolopyrrole (DPP) conjugated polymer, wherein the conjugated polymer DT-NO 2 Has the following structure:
[0042]
[0043] The above conjugated polymer DT-NO 2 The synthesis steps include: 2,5-bis(2-octyldodecyl)-3,6-bis(5-(trimethyltin)thiophen-2-yl)-2,5-dihydropyrrole Do[3,4-c]pyrrole-1,4-dione (0.1 g, 0.084 mmol), 4,7-bis(5-bromothiophen-2-yl)-5,6-dinitrobenzo[ c][1,2,5]thiadiazole (0.04 g, 0.084 mmol), Pd (PPh 3 ) 4 (10 mg, 0.0084 mmol) and 10 mL of toluene in N 2 It was added to a 50 mL preset reaction tube under the atmosphere, and the mixture was refluxed for 7 hours. After cooling to room temperature, the reaction solution was added dropwise to 200 mL methanol solution, and the mixture was collected by filtration under reduced pressure to obtain the final product DT-NO. 2 (the yield is 60%), and its hydrogen nuclear magnetic resonance spectrum is as follows Figure 5 As shown, the peak between 6.5-8.0 in the ...
Embodiment 3
[0051] A NIR-IIb fluorescent probe based on diketopyrrolopyrrole (DPP) conjugated polymer, wherein the conjugated polymer DT-F has the following structure:
[0052]
[0053] The synthesis steps of the above-mentioned conjugated polymer DT-F include: 2,5-bis(2-octyldodecyl)-3,6-bis(5-(trimethyltin)thiophene-2-yl) -2,5-Dihydropyrrolo[3,4-c]pyrrole-1,4-dione (0.1 g, 0.084 mmol), 4,7-bis(5-bromothienyl-2-)-5, 6-Difluoro-2,1,3-benzothiadiazole (0.04 g, 0.084 mmol), Pd (PPh 3 ) 4 (10 mg, 0.0084 mmol) and 10 mL of toluene in N 2 It was added into a 50 mL preset reaction tube under the atmosphere, and the mixture was refluxed for 7 h. After cooling to room temperature, the reaction solution was added dropwise to 200 mL methanol solution, and the mixture was collected by filtration under reduced pressure to obtain the final product DT-F (the yield was 52%). ), its synthetic route is as follows:
[0054]
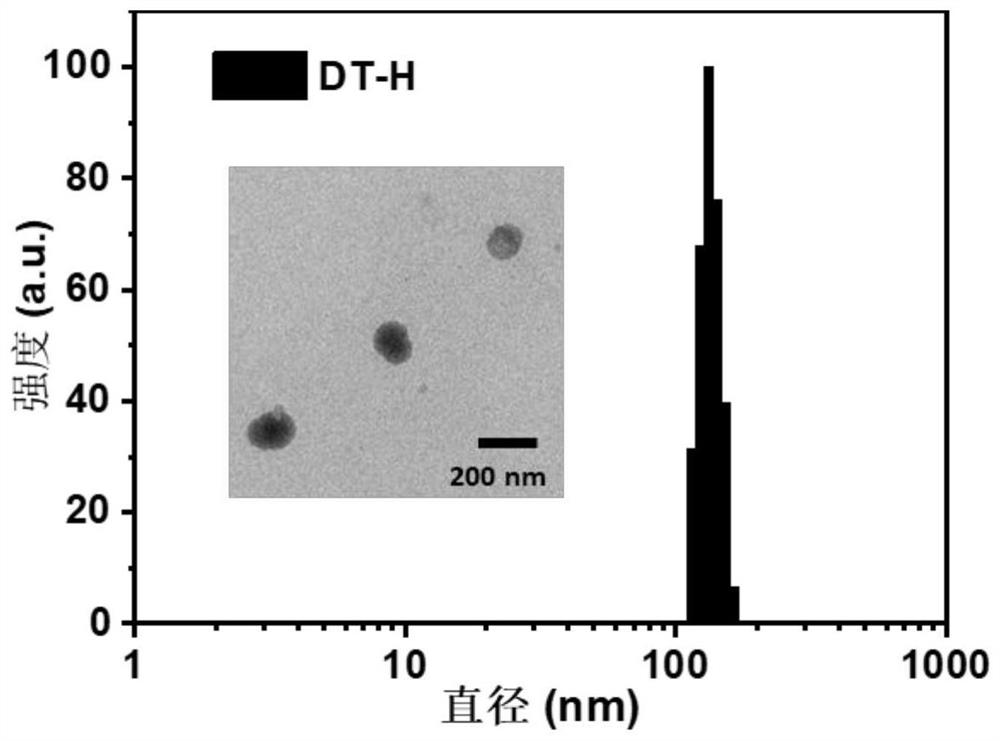
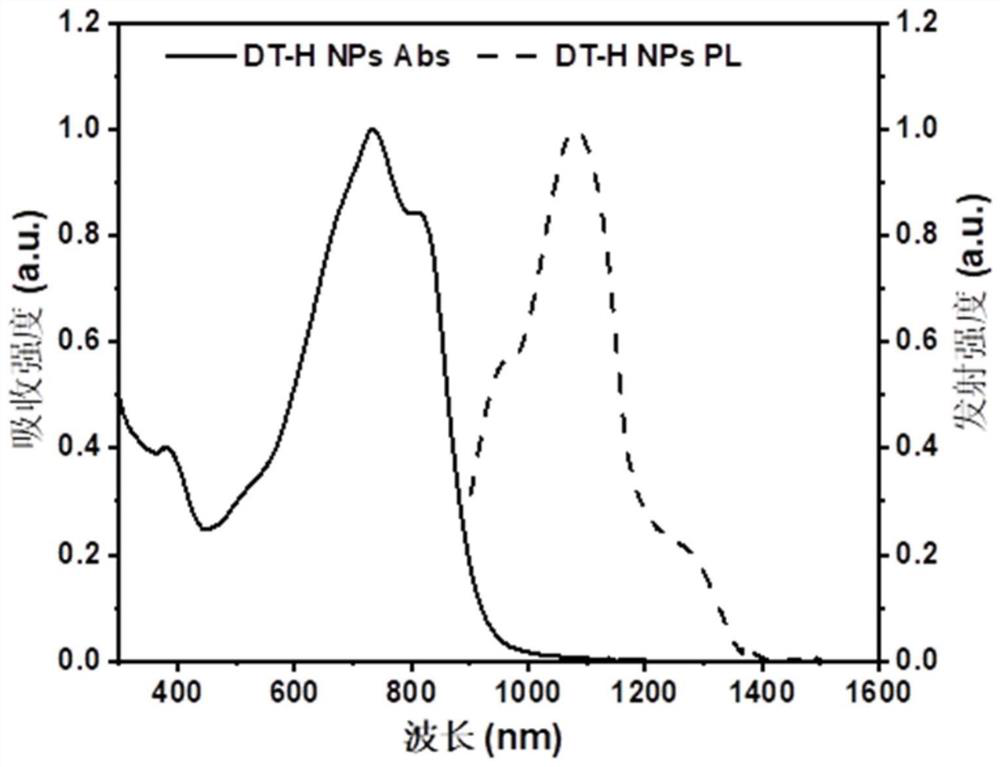
[0055] This embodiment provides a nanoparticle of the above-mentioned n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com