Drug target for treating cancer and application thereof
A technology of drugs and targets, applied in the field of biomedicine, to achieve good medicinal prospects and strong pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Synthesis of Microcolin H and derivatives
[0060] The invention decomposes the natural product Microcolin H into three fragments, M1, M2 and M3, respectively completes the synthesis of the three fragments M1, M2 and M3, then condenses the three fragments step by step, and finally obtains the natural product Microcolin H.
[0061]
[0062]
[0063] 1.1 Preparation of Microcolin H
[0064] 1) Preparation of M1:
[0065]
[0066] (R)-2-Methyloctanoic acid (M1)
[0067] Dissolve 3.5 g (11.6 mmol) of (R)-4-benzyl-3-octanoyloxazolidin-2-one (3) in 15 mL of THF, add 12.7 mL (12.7 mmol) of NaHMDS dropwise at -78°C, Stir for 30 min, then add 1.45 mL (23.2 mmol) MeI, and react at -78°C for 5 h. After the reaction was completed, it was quenched with saturated ammonium chloride, extracted three times with EtOAc (100 mL), and the organic phases were combined and 5% Na was used. 2 S 2 O 3 , extracted once with saturated brine, and purified by column chromato...
Embodiment 2
[0161] Example 2 Testing the activity of Microcolin H and derivative molecules on tumor cells
[0162] Various cancer cells were prepared into 50,000 cells / ml cell suspension, added to a 96-well plate cell culture dish, and the compounds synthesized in Example 1 were added respectively. Each test concentration was 6 wells, and set at 37°C, 5% CO. 2 After culturing for 48 hours under saturated humidity conditions, the absorbance A value was measured by the CCK8 method at a wavelength of 450 nm in an enzyme-linked detector, and the inhibitory effect of the compounds of the present invention on the test cancer cells was calculated.
[0163] Table 1 Inhibitory activity of Microcolin H on various tumor cells
[0164] cell HuH-7 HepaRG HGC-27 AGS MKN-28 A549 H460 AsPC-1 PANC-1 Hela IC 50 (nM)
1.3 5.7 3.5 8.6 50.2 0.7 10.1 3.5 2.5 132.6
[0165] The activity test shows that the marine polypeptide Microcolin H is effective on human l...
Embodiment 3
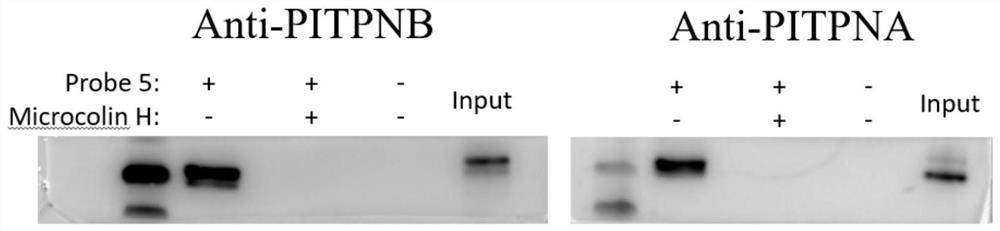
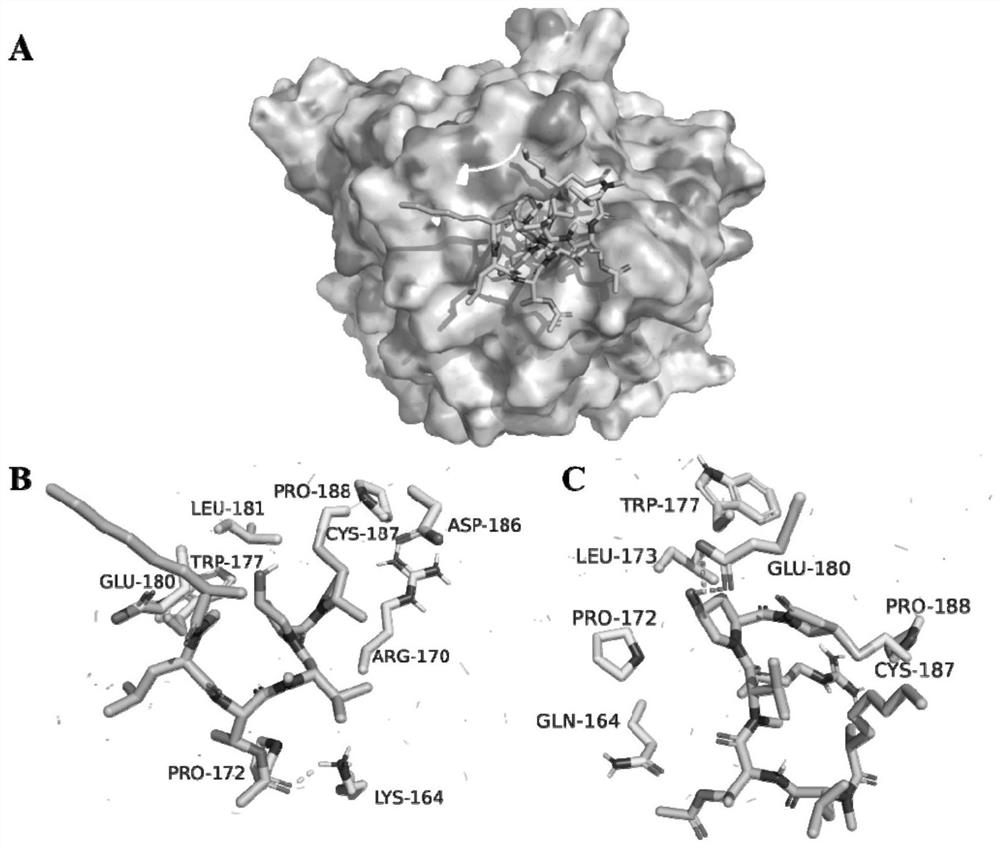
[0170] Example 3 Investigating the direct action target of Microcolin H in tumor cells
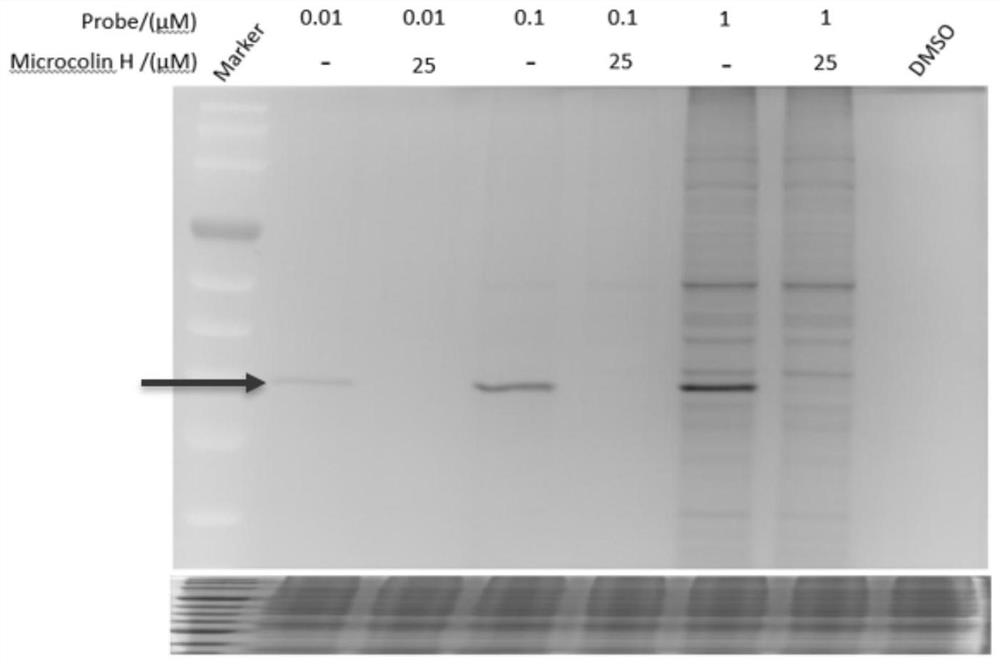
[0171] The present invention utilizes the probe molecule Probe 5 synthesized in Example 1 to incubate with cell lysate or living cells, and after incubation, the alkynyl part of the probe and the reagent rhodamine-azide undergo a "click chemistry" reaction, and the target that Microcolin H binds to The protein will have a rhodamine fluorescent label. After SDS-PAGE separation, the target protein of Microcolin H can be found by fluorescence imaging. The specific experimental process is as follows:
[0172] Different concentrations of probe molecules (probe 5 concentration: 0.01 μM, 0.1 μM, 1.0 μM) were incubated with gastric cancer cell HGC lysate, while the control group (25 μM concentration) was competed with 25 μM parent molecule Microcolin H as a cold probe, 1 After hours, the fluorescent reagents Rhodamine-azide (10 μM), 100 μM TBTA, 1 mM TCEP, and 1 mM CuSO were added 4 The Click rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com