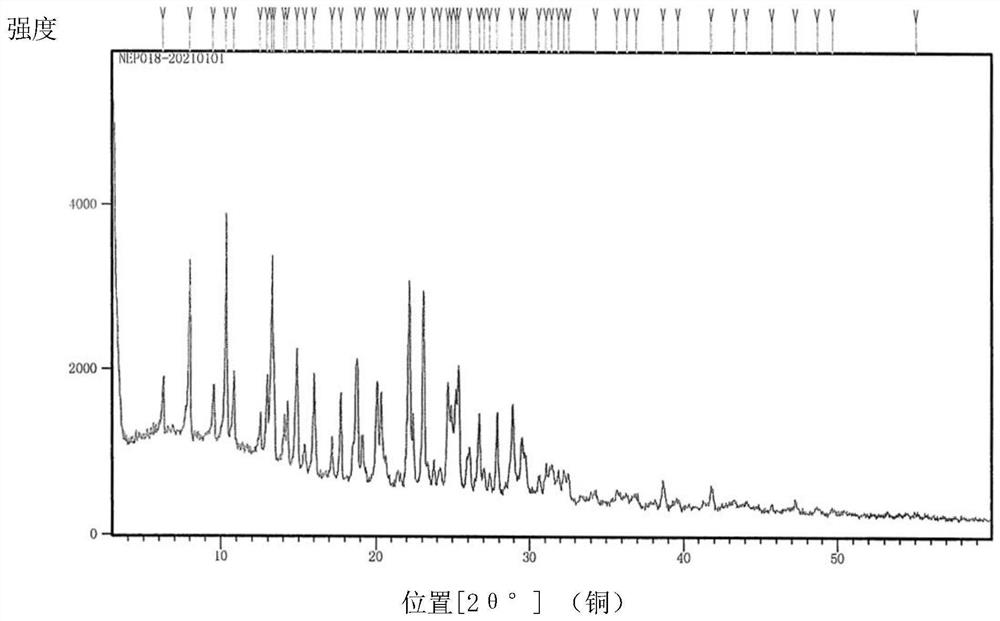
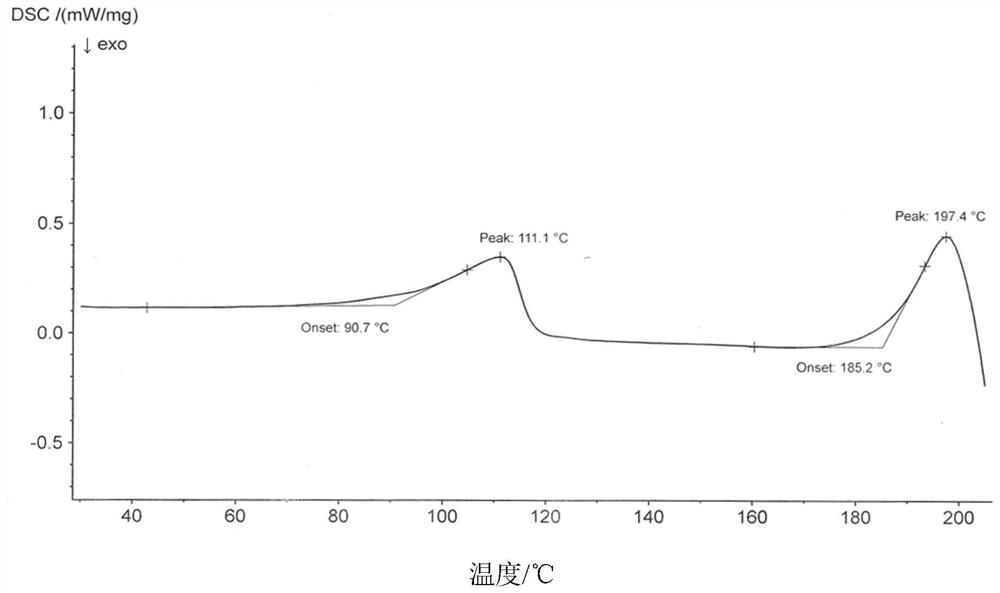
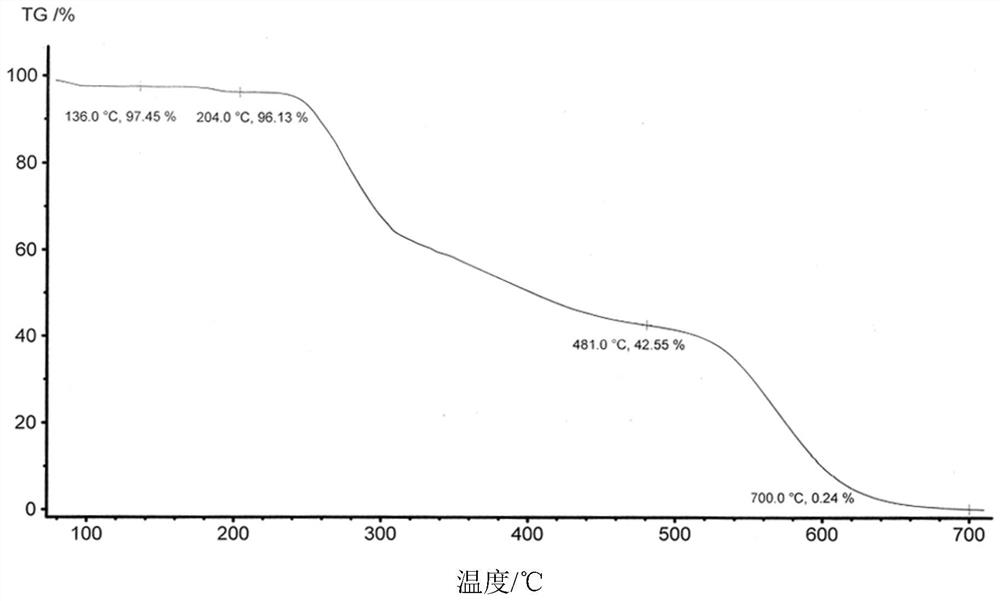
Crystal form A of thienopyridine compound, preparation method and pharmaceutical composition of thienopyridine compound
A compound and crystal form technology, applied in the field of medicine, can solve the problems of crystallization or purification of compounds that are not publicly displayed, and achieve the effects of easier quality control, high chemical stability, and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 100g of 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3, 2-b]Pyridin-2-yl)-1H-pyrazolyl-1-yl)ethyl valine ester hydrochloride crude product was added to the reaction flask, and 1000mL of a mixed solution of tetrahydrofuran and water (V tetrahydrofuran: V water = 9: 1), heated and refluxed to 80°C with stirring. After dissolving, stir for 30 minutes, then cool down to 15-25°C, stir and crystallize for 12h, filter with suction, and dry the filter cake to obtain 80 g of solid; add the obtained solid into the reaction flask, add 160ml of methanol, beating at 20-25°C for 4h, Suction filtration; the obtained filter cake was air-dried at 50° C. to obtain 76 g of white powder with a total yield of 76% in two steps. The resulting compound is 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3 ,2-b] pyridin-2-yl)-1H-pyrazolyl-1-yl) A crystal form of ethyl valine ester hydrochloride, it...
Embodiment 2
[0077] Weigh 100g of 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3, 2-b]Pyridin-2-yl)-1H-pyrazolyl-1-yl)ethyl valine ester hydrochloride crude product was added to the reaction flask, and 800mL of a mixed solution of methanol and water (V methanol: V water = 9:1), heated and refluxed to 70°C with stirring. After dissolving, stir for 30 minutes, then lower the temperature to 15-25°C, stir and crystallize for 12h, filter with suction, and dry the filter cake to obtain 75 g of solid; add the obtained solid into a reaction flask, add 150ml of methanol, and beat at 20-25°C for 4h, Suction filtration; the obtained filter cake was air-dried at 50° C. to obtain 71 g of white powder with a total yield of 71% in two steps. The resulting compound is 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3 ,2-b]Pyridin-2-yl)-1H-pyrazolyl-1-yl)ethylvaline ester hydrochloride A crystal form.
Embodiment 3
[0079] Weigh 100g of 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3, 2-b]Pyridin-2-yl)-1H-pyrazolyl-1-yl)ethyl valine ester hydrochloride crude product, added to the reaction flask, added 1500ml of tetrahydrofuran solution, heated and refluxed to 80 ℃ under stirring . After dissolving, stir for 30 minutes, then cool down to 15-25°C, stir and crystallize for 12h, suction filter, and dry the filter cake to obtain 85g of solid; add the obtained filter cake to the reaction flask, add 170ml of methanol, beating at 20-25°C for 4h , suction filtration; the obtained filter cake was air-dried at 50° C. to obtain white powder 81, and the two-step total yield was 81%. The resulting compound is 2-(4-(7-(2-fluoro-4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamide)phenoxy)thieno[3 ,2-b]Pyridin-2-yl)-1H-pyrazolyl-1-yl)ethylvaline ester hydrochloride A crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com