Method and kit for detecting existence or proportion of fetal free DNA in pregnant woman sample
A technology for samples and detection probes, applied in recombinant DNA technology, DNA/RNA fragments, biochemical equipment and methods, etc., can solve the problems of inability to integrate, high sequencing cost, difficult clinical popularization, etc. The effect of high degree of automation and short detection cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
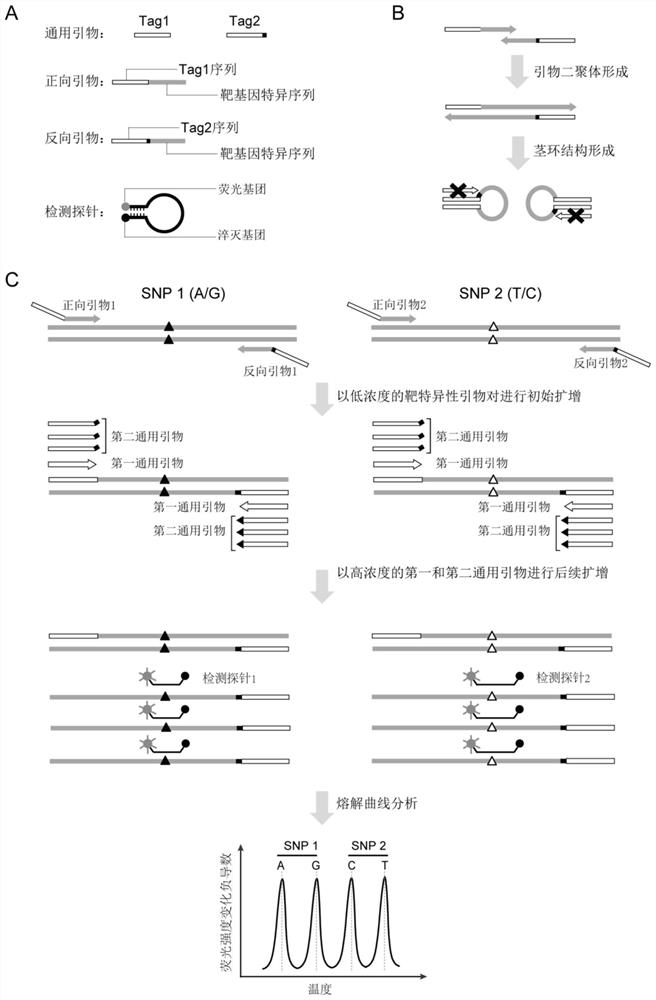
[0213] Embodiment 1. Selection of candidate SNP sites
[0214] The SNP site of the present application is selected from the single nucleotide polymorphism site library (dbSNP) of the National Center for Biotechnology Information (NCBI) in the United States, and the preferred candidate SNP site meets the following conditions: (1) between different races Fst (population fixation coefficient) 1Mb; (5) In order to avoid linkage between different sites, try to select sites on different chromosomes . The present embodiment selects 23 candidate SNP sites according to the above preferred standards, specifically as shown in Table 1, SNP site information and sequences are queried and downloaded from the dbSNP database of the National Center for Biotechnology Information (NCBI), the alleles The frequency refers to the Asian population frequency from the Thousand Genomes database, and these loci are evenly distributed on each chromosome of the genome.
[0215] Table 1. Candidate SNP s...
Embodiment 2
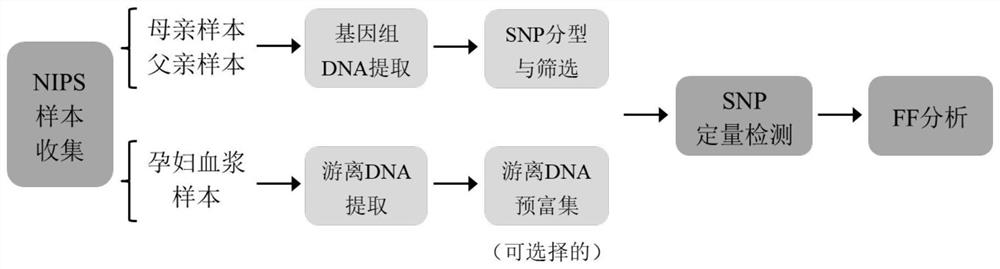
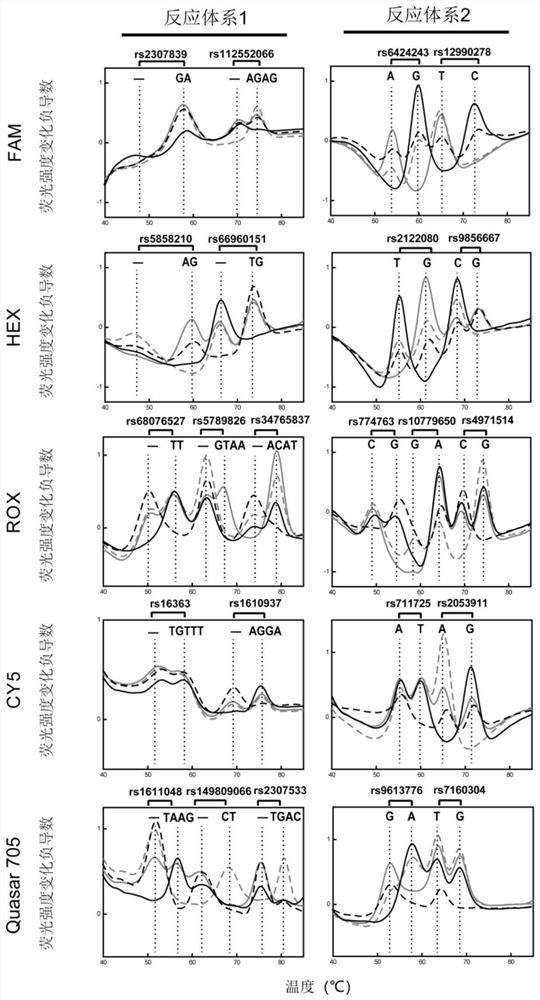
[0217] Example 2. Detection of Fetal Free DNA Ratio in Noninvasive Prenatal Screening Samples
[0218] In this example, 4 cases of non-invasive prenatal screening samples were collected and detected in the clinic, and the detection ability of the method of the present invention was investigated. The overall experimental process is as follows: figure 2 shown.
[0219] 3.1 Collection and extraction of samples
[0220] Four groups of non-invasive prenatal screening samples were collected, and each group of samples included peripheral blood samples from pregnant women (8-14 weeks of pregnancy) and saliva samples from corresponding parents of the fetus (hereinafter referred to as "mother's sample" and "father's sample" respectively). Saliva samples were collected according to the instructions of the saliva collector (Xiamen Zhishan Biotechnology Co., Ltd., Xiamen), and stored at room temperature. Use EDTA anticoagulant tubes (Zhejiang Gongdong Medical Instrument Co., Ltd., Tai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com