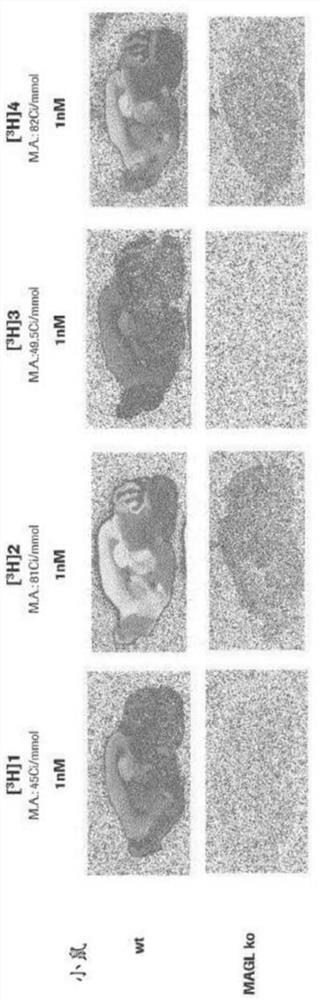
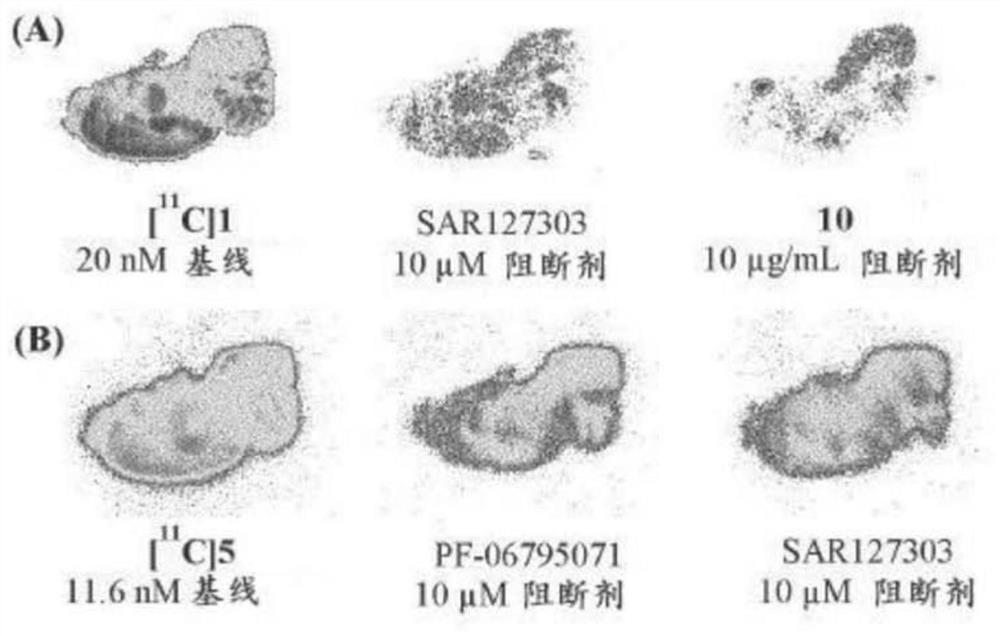
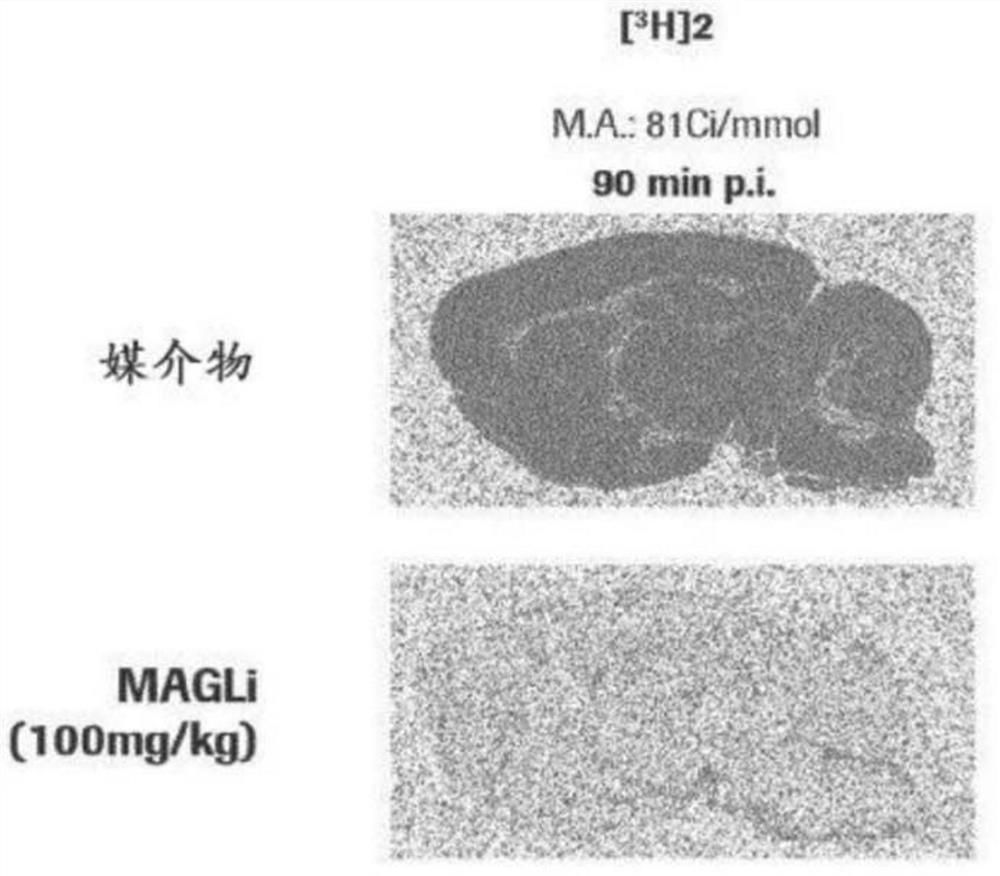
Radiolabelled compounds
A technology for radioactive labeling and compounds, applied in the direction of in vivo radioactive preparations, introduction of isotopes of heterocyclic compounds, introduction of isotopes into organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0112] The present invention will be more fully understood by reference to the following examples. However, the claims should not be construed as limiting the scope of the embodiments.
[0113] The following abbreviations are used in this text:
[0114] AcOH=acetic acid, ACN=acetonitrile, BEMP=2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphine, Boc= tert-Butoxycarbonyl, CAS RN=Chemical Abstracts Registration Number, Cbz=benzyloxycarbonyl, Cs 2 CO 3 = cesium carbonate, CO = carbon monoxide, CuCl = cuprous(I) chloride, CuCN = cuprous(I) cyanide, CuI = cuprous(I) iodide, DMAP = 4-dimethylaminopyridine, DME = Dimethoxyethane, DMEDA=N,N'-dimethylethylenediamine, DMF=N,N-dimethylformamide, DIPEA=N,N-diisopropylethylamine, dppf=1, 1-Bis(diphenylphosphino)ferrocene, EDC.HCl=N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride, EI=electron bombardment, ESI = Electrospray ionization, EtOAc = ethyl acetate, EtOH = ethanol, h = hours, FA = formic acid,...
example 1
[0130] (4aR,8aS)-6-[3-[2-[2-Fluoro-6-(trifluoromethyl)phenyl]ethyl]azetidine-1-carbonyl]-4,4a,5, 7,8,8a-Hexahydropyrido[4,3-b][1,4]oxazin-3-one
[0131]
[0132] To (4aR,8aS)-3-oxohexahydro-2H-pyrido[4,3-b][1,4]oxazine-6(5H)-carboxylic acid 4-nitrophenyl ester (BB2) (42 mg , 131 μmol) and DIPEA (42.3 mg, 57.1 μL, 327 μmol) in ACN (0.5 mL) was added 3-(2-fluoro-4-(trifluoromethyl)phenethyl)azetidine 4 - Tosylate (60.3 mg, 144 μmol) and the yellow solution was stirred at RT overnight. To the reaction solution was added silica gel, and the mixture was evaporated. The compound was purified by silica gel chromatography using an MPLC system on a 4 g chromatography column and eluting with a gradient of n-heptane:EtOAc / ethanol 3 / 1 (70:30 to 10:90). Using ACN:H on preparative HPLC (Gemini NX column) 2 A secondary purification with O (containing 0.1% formic acid) (20:80 to 98:2) gave the title compound as a colorless foam (0.038 g, 68%). MS(ESI): m / z=430.2[M+H] + .
[0133] In...
example 3H
[0146] instance[ 3 H]1
[0147] [ 3 H](4aR,8aS)-6-[3-[2-[2-fluoro-6-(trifluoromethyl)phenyl]ethyl]azetidine-1-carbonyl]-4,4a, 5,7,8,8a-Hexahydropyrido[4,3-b][1,4]oxazin-3-one
[0148]
[0149]In a 4 mL tritiated flask, place (4aR, 8aS)-6-(3-((E)-2-fluoro-6-(trifluoromethyl)styryl)azetidine-1-carbonyl) Hexahydro-2H-pyrido[4,3-b][1,4]oxazin-3(4H)-one (2.0 mg, 4.68 μmol) and palladium on activated carbon (10%) (4.98 mg, 4.68 μmol) Mix in ethanol (1 mL). The flask was connected to an RC Tritec tritium manifold and degassed by three freeze-thaw cycles. Tritium gas was introduced and the suspension was vigorously stirred at 610 mbar for 4 h under a tritium gas atmosphere. The solution was cooled with liquid nitrogen and the excess tritium gas in the reaction vessel was reabsorbed in a uranium trap for spent tritium. The solvent was lyophilized and the labile tritium was removed by lyophilization using MeOH (3 x 0.5 mL). The remaining black residue was suspended in ethanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com