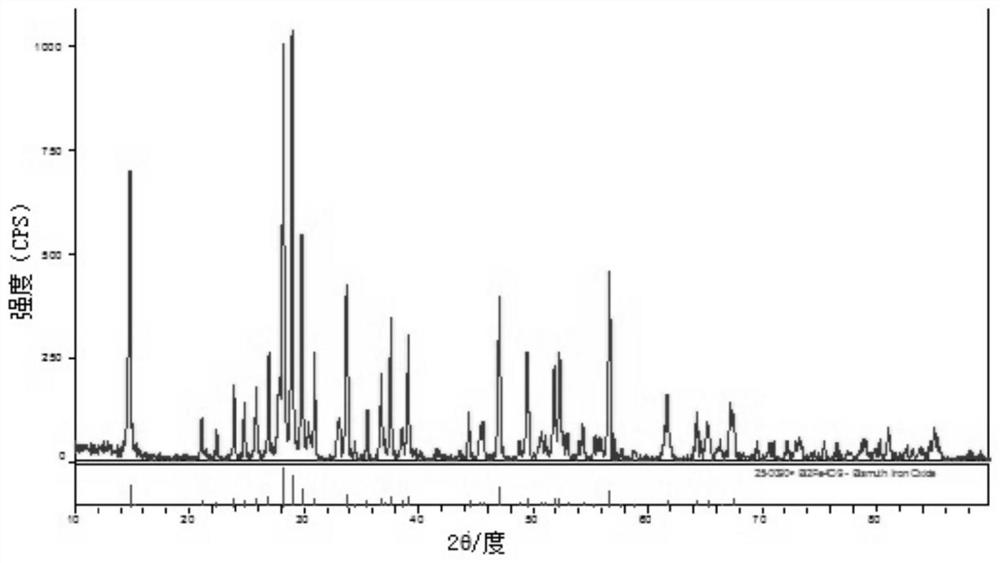
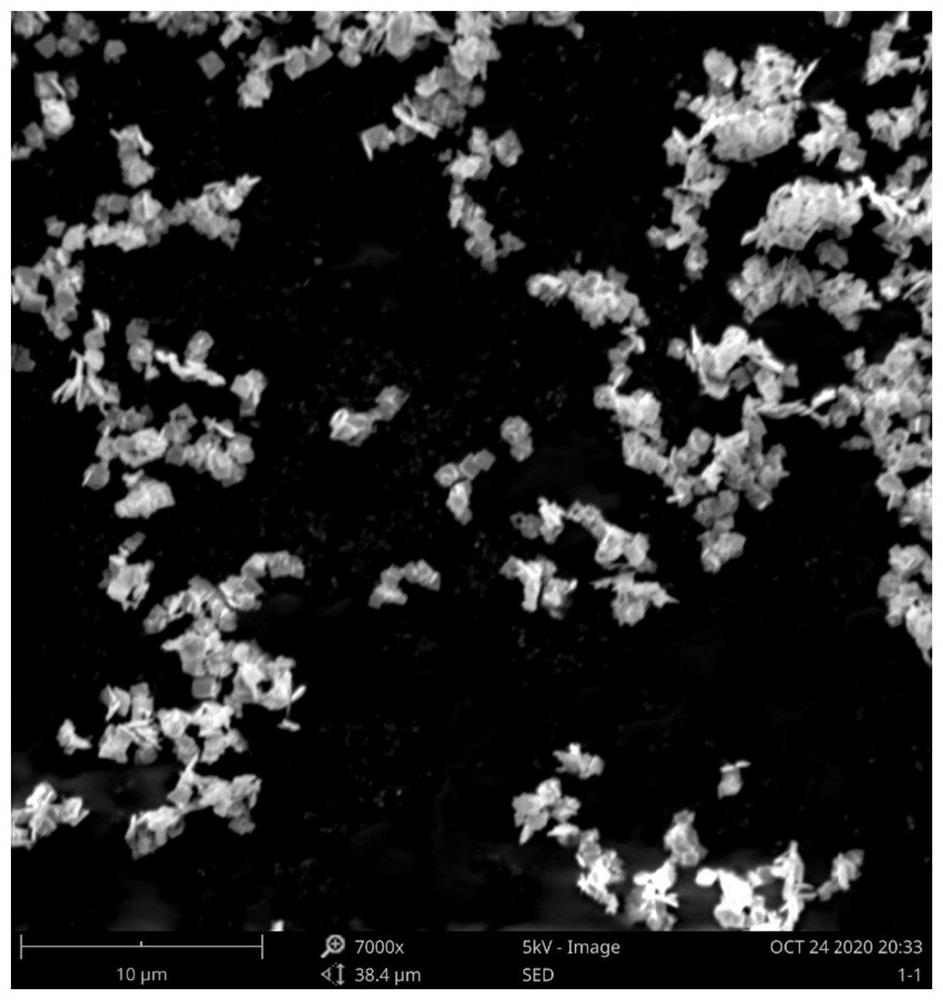
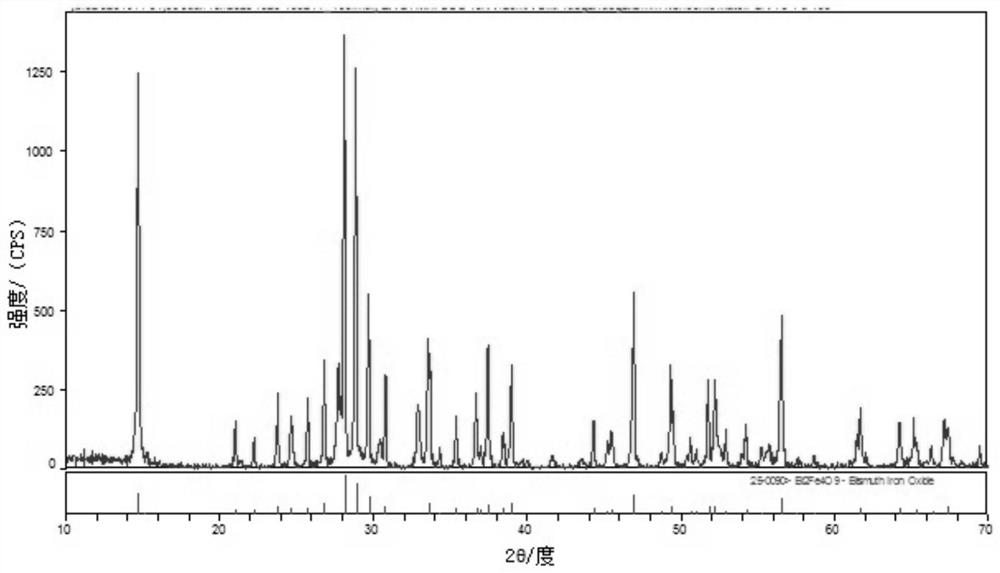
Bismuth ferrite nanosheet material as well as preparation method and application thereof
A technology of nanosheets and bismuth salts, applied in the field of environmental engineering, to achieve the effect of large particle size, small particle size, and large relative area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Analytically pure Bi(NO 3 ) 3 ·5H 2 O and FeCl 3 ·6H 2 O was dissolved in the ethylene glycol solution at a molar ratio of 1:1 and stirred well until completely dissolved. 200 mL of deionized water was added to the above solution, stirred, and then excess concentrated ammonia water was added dropwise to completely precipitate the solution. The supernatant was poured out, deionized water was added to the obtained precipitate, shaken, washed and centrifuged, and the above washing operation was repeated 2-3 times. 10 mol / L NaOH was added to the washed precipitate, stirred for 30 min to form a solution and put into a stainless steel autoclave with a polytetrafluoroethylene lining, and reacted in an oven at 180 °C for 72 h. After the reaction, turn off the power of the oven and let the autoclave naturally cool to room temperature in the oven. The final product of the reaction was washed 3-5 times with deionized water and absolute ethanol, and dried in an oven at 60 °C ...
Embodiment 2
[0027] Analytically pure Bi(NO 3 ) 3 ·5H 2 O and FeCl 3 ·6H 2O was dissolved in the ethylene glycol solution at a molar ratio of 1:1 and stirred well until completely dissolved. 200 mL of deionized water was added to the above solution, stirred, and then excess concentrated ammonia water was added dropwise to completely precipitate the solution. The supernatant was poured out, deionized water was added to the obtained precipitate, shaken, washed and centrifuged, and the above washing operation was repeated 2-3 times. 14mol / L NaOH was added to the washed precipitate, stirred for 30min to form a solution and put into a stainless steel autoclave with a polytetrafluoroethylene lining, and reacted in an oven at 180°C for 72h. After the reaction, turn off the power of the oven and let the autoclave naturally cool to room temperature in the oven. The final product of the reaction was washed 3-5 times with deionized water and absolute ethanol, and dried in an oven at 60 °C for 1...
Embodiment 3
[0029] Analytically pure Bi(NO 3 ) 3 ·5H 2 O and FeCl 3 ·6H 2 O was dissolved in the ethylene glycol solution at a molar ratio of 0.7:1 and stirred well until completely dissolved. 200 mL of deionized water was added to the above solution, stirred, and then excess concentrated ammonia water was added dropwise to completely precipitate the solution. The supernatant was poured out, deionized water was added to the obtained precipitate, shaken, washed and centrifuged, and the above washing operation was repeated 2-3 times. 10 mol / L NaOH was added to the washed precipitate, stirred for 30 min to form a solution and put into a stainless steel autoclave with a polytetrafluoroethylene lining, and reacted in an oven at 180 °C for 72 h. After the reaction, turn off the power of the oven and let the autoclave naturally cool to room temperature in the oven. The final product of the reaction was washed 3-5 times with deionized water and anhydrous ethanol, and dried in an oven at 60 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com