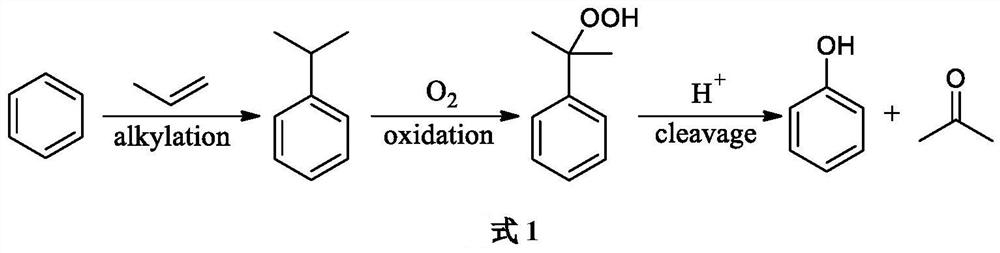
Improved method for producing phenol/acetone
A technology of acetone and phenol, which is applied in the field of phenol/acetone production, can solve problems such as overheating, achieve the effects of avoiding local overheating, efficient heat transfer, and ensuring decomposition stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] At a reaction temperature of 100 ° C, the addition of cumene hydrogen peroxide (CHP) initiator was 5%, and under the condition of vigorous stirring, oxygen was continuously fed into the 1000 mL cumene reaction solution, and the oxygen flow rate was 80 mL / min, After 8 hours of reaction, a cumene oxidation reaction solution with cumene hydrogen peroxide (CHP) concentration of 24.6%, cumene concentration of 71.5% and dimethylbenzyl alcohol (DMPC) content of 2.5% was obtained.
[0031] Take the cumene oxidation reaction solution obtained as raw material, realize the full mixing of raw material and acid solution in micro-mixer, the mass concentration of the acetone solution of sulfuric acid is 2%, and control flow makes sulfuric acid consumption be cumene hydrogen peroxide ( CHP) of 2.0‰. The mixed raw materials enter into a tubular reactor for reaction. The inner diameter of the tubular reactor is 26 mm and filled with Pall rings; the reaction temperature is 110 °C, and the...
Embodiment 2
[0034] At a reaction temperature of 100 ° C, the addition of cumene hydrogen peroxide (CHP) initiator was 5%, and under the condition of vigorous stirring, oxygen was continuously fed into the 1000 mL cumene reaction solution, and the oxygen flow rate was 80 mL / min, After 5 hours of reaction, a cumene oxidation reaction solution with cumene hydrogen peroxide (CHP) concentration of 19.5%, cumene concentration of 77.6% and dimethylbenzyl alcohol (DMPC) content of 2.0% was obtained.
[0035] Take the cumene oxidation reaction solution obtained as raw material, realize the full mixing of raw material and acid solution in micro-mixer, the mass concentration of the acetone solution of sulfuric acid is 2%, and control flow makes sulfuric acid consumption be cumene hydrogen peroxide ( CHP) of 2.0‰. The mixed raw materials enter into a tubular reactor for reaction. The inner diameter of the tubular reactor is 26 mm and filled with Pall rings; the reaction temperature is 140 °C, and the...
Embodiment 3
[0038] At a reaction temperature of 100 ° C, the addition of cumene hydrogen peroxide (CHP) initiator was 8%, and under the condition of vigorous stirring, oxygen was continuously fed into the 1000 mL cumene reaction solution, and the oxygen flow rate was 80 mL / min, After 6 hours of reaction, a cumene oxidation reaction solution with cumene hydrogen peroxide (CHP) concentration of 32.8%, cumene concentration of 60.3% and dimethylbenzyl alcohol (DMPC) content of 3.3% was obtained.
[0039] Taking the obtained cumene oxidation reaction liquid as the raw material, the raw material and the acid solution are fully mixed in the micro-mixer, the mass concentration of the acetone solution of methanesulfonic acid is 1%, and the flow rate is controlled so that the amount of methanesulfonic acid is over 1%. 5.0‰ of cumene hydrogen oxide (CHP). The mixed raw materials enter into a tubular reactor for reaction. The inner diameter of the tubular reactor is 26 mm and filled with Pall rings; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com